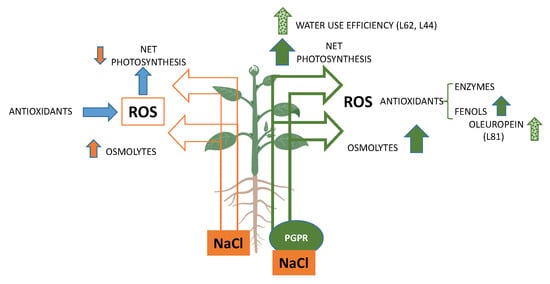
Management of Plant Physiology with Beneficial Bacteria to Improve Leaf Bioactive Profiles and Plant Adaptation under Saline Stress in Olea europea L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beneficial Strains and Olive Tree Variety
2.2. Inocula Preparation and Delivery to Plants
2.3. Experimental Design
2.4. Photosynthesis (Chlorophyll Fluorescence)
2.5. Photosynthesis (CO2 Fixation)
2.6. Photosynthetic Pigments: Chlorophylls and Carotenoids
2.7. Enzymatic Antioxidants: Superoxide Dismutase (SOD) and Ascorbate Peroxidase (APX)
2.8. Osmolites: Proline and Soluble Sugars
2.9. Total Phenols and Flavonols
2.10. Oleuropein Extraction and TLC Analysis
2.11. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vossen, P. Olive Oil: History, Production, and Characteristics of the World’s Classic Oils. Hortscience 2007, 42, 1093–10100. [Google Scholar] [CrossRef] [Green Version]
- Tous, J.; Romero, A.; Hermoso, J.J. New trends in olive orchard design for continuous mechanical harvesting. Adv. Hort. Sci. 2010, 24, 43–52. [Google Scholar]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Immediate responses and adaptive strategies of three olive cultivars under contrasting water availability regimes: Changes on structure and chemical composition of foliage and oxidative damage. Plant Sci. 2006, 170, 596–605. [Google Scholar] [CrossRef]
- Mousavi, S.; Regni, L.; Bocchini, M.; Mariotti, R.; Cultrera, N.G.M.; Mancuso, S.; Googlani, J.; Chakerolhosseini, M.R.; Guerrero, C.; Albertini, E.; et al. Physiological, epigenetic and genetic regulation in some olive cultivars under salt stress. Sci. Rep. 2019, 9, 1093. [Google Scholar] [CrossRef] [Green Version]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Boyer, J.S.; Armond, P.A.; Sharp, P.E. Light stress and leaf water relations. In Photoinhibition, Topics in Photo-Synthesis; Kyle, D.J., Osmond, C.D., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 9, pp. 111–122. [Google Scholar]
- Chaves, M.M.; Pereira, J.S.; Maroco, J. Understanding plant response to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 1–26. [Google Scholar] [CrossRef]
- Apel, K.; Hirth, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Grace, S.C. Phenolics as antioxidants. In Antioxidants and Reactive Oxygen Species in Plants; Smirnoff, N., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2005; pp. 141–168. [Google Scholar]
- BenAhmed, C.; BenRouina, B.; Sensoy, S.; Boukhris, M.; BenAbdallah, F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci. 2004, 166, 293–302. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.M.; Khemira, H. Osmoregulation and osmoprotection in the leaf cells of two olive cultivars subjected to severe water deficit. Acta Physiol. Plant. 2009, 31, 711–721. [Google Scholar] [CrossRef]
- Oh, M.; Trick, H.N.; Rajashekar, C.B. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; León, L.; Rallo, L.; Luque de Castro, M.L. Hydrophilic antioxidants of virgin olive oil. Part 1: Hydrophilic phenols: A key factor for virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2011, 113, 678–691. [Google Scholar] [CrossRef]
- Bulotta, S.; Oliverio, M.; Russo, D.; Procopio, A. Biological activity of oleuropein and its derivatives. In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3605–3638. [Google Scholar]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Celano, M.; Maggisano, V.; Lepore, S.M.; Russo, D.; Bulotta, S. Secoiridoids of olive and derivatives as potential coadjuvant drugs in cancer: A critical analysis of experimental studies. Pharmacol. Res. 2019, 142, 77–86. [Google Scholar] [CrossRef]
- Pehlivan, N.; Sun, L.; Jarrett, P.; Yang, X.; Mishra, N.; Chen, L.; Kadioglu, A.; Shen, G.; Zhang, H. Co-overexpressing a plasma membrane and a vacuolar membrane Sodium/Proton antiporter significantly improves salt tolerance in transgenic Arabidopsis plants. Plant Cell Physiol. 2016, 57, 1069–1084. [Google Scholar] [CrossRef]
- Yasmin, F.; Biswas, S.; Jewel, G.; Elias, S.; Seraj, Z. Constitutive overexpression of the plasma membrane Na+/H+ antiporter for conferring salinity tolerance in rice. Plant Tissue Cult. Biotechnol. 2016, 25, 257–272. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Schroth, M.N.; Miller, T.D. Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 1980, 70, 1078–1082. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Reddy, M.S.; Al-Turki, A.I. Recent Trends in PGPR Research for Sustainable Crop Productivity; Scientific Publishers: Jodhpur, India, 2016; p. 258. ISBN 978-81-7233-990-6. [Google Scholar]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil. 2018, 428, 35–55. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Seco, D.; Zhang, Y.; Gutierrez-Mañero, F.J.; Martin, C.; Ramos-Solano, B. Application of Pseudomonas fluorescens to Blackberry under Field Conditions Improves Fruit Quality by Modifying. Flavonoid Metabolism. PLoS ONE. 2015, 10, e0142639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algar, E.; Ramos-Solano, B.; García-Villaraco, A.; Saco Sierra, M.D.; Martín Gómez, M.S. Gutiérrez-Mañero, F.J. Bacterial Bioeffectors Modify Bioactive Profile and Increase Isoflavone Content in Soybean Sprouts (Glycine max var Osumi). Plant Foods Hum. Nutr. 2013, 68, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Garcia-cristobal, J.; García-Villaraco, A.; Ramos, B.; Gutierrez-Mañero, J.; Lucas, J.A. Priming of pathogenesis related-proteins and enzymes related tooxidative stress by plant growth promoting rhizobacteria on riceplants upon abiotic and biotic stress challenge. J. Plant Physiol. 2015, 188, 72–79. [Google Scholar] [CrossRef]
- Lucas, J.A.; García-Cristobal, J.; Bonilla, A.; Ramos, B.; Gutierrez-Mañero, J. Beneficial rhizobacteria from rice rhizosphere confers high protection against biotic and abiotic stress inducing systemic resistance in rice seedlings. Plant Physiol. Biochem. 2014, 82, 44–53. [Google Scholar] [CrossRef]
- Barriuso, J.; Ramos Solano, B.; Gutiérrez Mañero, F.J. Protection Against Pathogen and Salt Stress by Four Plant Growth-Promoting Rhizobacteria Isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 2008, 98, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Mañero, F.J.; García-Villaraco, A.; Lucas, J.A.; Gutiérrez, E.; Ramos-Solano, B. Inoculant/Elicitation Technology to Improve Bioactive/Phytoalexin Contents in Functional Foods. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 224–241. [Google Scholar]
- Barriuso, J.; Pereyra, M.T.; García, J.A.L.; Megías, M.; Gutierrez-Mañero, F.J.; Ramos-Solano, B. Screening for putative PGPR to improve establishment of the symbiosis Lactarius deliciosus-Pinus sp. Microb. Ecol. 2005, 50, 82–89. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schlosser, A.J.; Martin, J.M.; Hannah, L.C.; Giroux, M.J. The maize leaf starch mutation has diminished field growth and productivity. Crop Sci. 2012, 52, 700–706. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- García-Limones, C.; Hervas, A.; Navas-Cortes, J.A.; Jimenez-Díaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Yemm, E.M.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- TerBraak, C.F.J.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Centre for Biometry: Wageningen, The Netherlands, 1998. [Google Scholar]
- Chartzoulakis, K. Salinity and olive: Growth, salt tolerance, photosynthesis and yield. Agric. Water Manag. 2005, 78, 108–121. [Google Scholar] [CrossRef]
- Angelopoulos, K.; Dichio, B.; Xiloyannis, C. Inhibition of photosynthesis in olive trees (Olea europaea L.) during water stress and rewatering. J. Exp. Bot. 1996, 47, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Bongi, G.; Loreto, F. Gas-exchange properties of salt-stressed olive (Olea europaea L.) leaves. Plant Physiol. 1989, 90, 1408–1413. [Google Scholar] [CrossRef] [Green Version]
- Evans, G.R.; Von Caemmerer, S.; Setchell, B.A.; Hudson, G.S. The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Austr. J. Plant Physiol. 1994, 21, 475–495. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Lloyd, J.; McConchie, C.; Kriedemann, P.E.; Farquhar, G.D. On the site of biophysical constrains to CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ. 1995, 18, 149–157. [Google Scholar] [CrossRef]
- Tattini, M.; Gucci, R.; Coradeschi, M.A.; Ponzio, C.; Everarard, J.D. Growth, gas exchange and ion content in Olea europaea plants during salinity and subsequent relief. Physiol. Plantarum. 1995, 95, 203–210. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Loupassaki, M.; Bertaki, M.; Androulakis, I. Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. Sci. Horticult. 2002, 96, 235–247. [Google Scholar] [CrossRef]
- Pérez-Alfocea, F.; Albacete, A.; Ghanem, M.E.; Dodd, I.C. Hormonal regulation of source-sink relations to maintain crop productivity under salinity: A case study of root-to-shoot signalling in tomato. Funct. Plant Biol. 2010, 37, 592–603. [Google Scholar] [CrossRef]
- Rejsková, A.; Patková, L.; Stodulková, E.; Lipavská, H. The effect of abiotic stresses on carbohydrate status of olive shoots (Olea europaea L.) under in vitro conditions. J. Plant Physiol. 2007, 164, 174–184. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Gutierrez-Albanchez, E.; Kirakosyan, A.; Bolling, S.F.; García-Villaraco, A.; Gutierrez-Mañero, J.; Ramos-Solano, B. Biotic elicitation as a tool to improve strawberry and raspberry extract potential on metabolic syndrome-related enzymes in vitro. J. Sci. Food Agric. 2019, 99, 2939–2946. [Google Scholar] [CrossRef]
- Boue, S.M.; Cleveland, T.E.; Carter-Wientjes, C.; Shih, B.Y.; Bhatnagar, D.; Mclachlan, J.M.; Burow, M.E. Phytoalexin-Enriched Functional Foods. J. Agric. Food Chem. 2009, 57, 2614–2622. [Google Scholar] [CrossRef]
- Koudounas, K.; Banilas, G.; Michaelidis, C.; Demoliou, C.; Rigas, S.; Hatzopoulos, P. A defence-related Olea europaea β-glucosidase hydrolyses and activates oleuropein into a potent protein cross-linking agent. J. Exp. Bot. 2015, 66, 2093–2106. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Utrilla, J.; Ferro-García, M.A.; Mingorance, M.D.; Bautista-Toledo, I. Adsorption of lead on activated carbons from olive stones. J. Chem. Technol. Biotechnol. 1986, 36, 47–52. [Google Scholar] [CrossRef]
- Galiatsatou, P.; Metaxas, M.; Kasselouri-Rigopoulou, V. Mesoporous activated carbon from agricultural byproducts. Mikrochim. Acta. 2001, 136, 147–152. [Google Scholar] [CrossRef]
- Khalil, L.B.; Girgis, B.S.; Tawfik, T. Decomposition of H2O2 on activated carbon obtained from olive stones. J. Chem. Technol. Biotechnol. 2001, 76, 1132–1140. [Google Scholar] [CrossRef]
- Blázquez, G.; Hernainz, F.; Calero, M.; Ruiz-Nuñez, L.F. Removal of cadmium ions with olive stones: The effect of some parameters. Proc. Biochem. 2005, 40, 2649–2654. [Google Scholar] [CrossRef]
- Ortega-Jurado, A.; Palomar-Carnicero, J.M.; Cruz-Peragon, F. Integral olive kernel elaboration with electricity generation compared with actual system of obtaining olive virgin oil. Grasas y Aceites 2004, 55, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Vlyssides, A.G.; Loizides, M.; Karlis, P.K. Integrated strategic approach for reusing olive oil extraction. J. Clean. Prod. 2004, 12, 603–611. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galicia-Campos, E.; Ramos-Solano, B.; Montero-Palmero, M.B.; Gutierrez-Mañero, F.J.; García-Villaraco, A. Management of Plant Physiology with Beneficial Bacteria to Improve Leaf Bioactive Profiles and Plant Adaptation under Saline Stress in Olea europea L. Foods 2020, 9, 57. https://doi.org/10.3390/foods9010057
Galicia-Campos E, Ramos-Solano B, Montero-Palmero MB, Gutierrez-Mañero FJ, García-Villaraco A. Management of Plant Physiology with Beneficial Bacteria to Improve Leaf Bioactive Profiles and Plant Adaptation under Saline Stress in Olea europea L. Foods. 2020; 9(1):57. https://doi.org/10.3390/foods9010057
Chicago/Turabian StyleGalicia-Campos, Estrella, Beatriz Ramos-Solano, Mª. Belén Montero-Palmero, F. Javier Gutierrez-Mañero, and Ana García-Villaraco. 2020. "Management of Plant Physiology with Beneficial Bacteria to Improve Leaf Bioactive Profiles and Plant Adaptation under Saline Stress in Olea europea L." Foods 9, no. 1: 57. https://doi.org/10.3390/foods9010057
APA StyleGalicia-Campos, E., Ramos-Solano, B., Montero-Palmero, M. B., Gutierrez-Mañero, F. J., & García-Villaraco, A. (2020). Management of Plant Physiology with Beneficial Bacteria to Improve Leaf Bioactive Profiles and Plant Adaptation under Saline Stress in Olea europea L. Foods, 9(1), 57. https://doi.org/10.3390/foods9010057