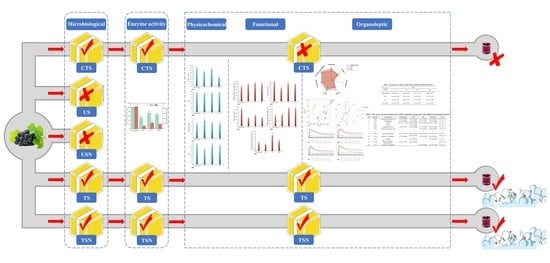
Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grape Juice Preparation
2.3. Nisin Solution Preparation
2.4. Sterilization Process
2.5. Microbiological Assay
2.6. Enzyme Activity Assay
2.7. Physicochemical Indicators
2.8. Functional Indicators
2.8.1. Determination of Total Polyphenol Content (TPC), Total Anthocyanin Content (TAC), and Total Flavonoid Content (TFC)
2.8.2. Antioxidant Capacity Assay
2.9. Organoleptic Properties
2.9.1. Color Measurement
2.9.2. Sensory Evaluation
2.9.3. Electronic Nose (E-Nose) Assay
2.9.4. HS-SPME-GC-MS Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Lethal Effects of Different Sterilization Treatments on the Microorganisms
3.1.1. TBC and Escherichia coli Counts
3.1.2. Yeast and Mold Counts
3.2. Passivation Effects of Different Sterilization Treatments on the Enzyme Activity
3.3. Effects of Different Sterilization Treatments on the Physicochemical Properties
3.3.1. TSS and pH Analysis
3.3.2. BI
3.3.3. Viscosity
3.4. Effects of Different Sterilization Treatments on the Functional Properties
3.4.1. TPC
3.4.2. TAC and TFC
3.4.3. Antioxidant Activity
3.5. Correlation Analysis of Antioxidant Activity
3.6. Effects of Different Sterilization Treatments on the Sensory Quality
3.6.1. Color Analysis
3.6.2. Artificial Sensory Evaluation
3.6.3. E-Nose Analysis
3.6.4. GC-MS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, C.K.; Ndiaye, M.A.; Mintie, C.A.; Chhabra, G.; Ahmad, N. Abstract 5263: Chemopreventive effects of dietary grapes on skin cancer. Cancer Res. 2017, 77, 5263. [Google Scholar] [CrossRef]
- Karacabey, E.; Mazza, G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010, 119, 343–348. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van De Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Vinson, J.A.; Teufel, K.; Wu, N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis 2001, 156, 67–72. [Google Scholar] [CrossRef]
- Hasan, M.; Yun, H.-K.; Kwak, E.-J.; Baek, K.-H. Preparation of resveratrol-enriched grape juice from ultrasonication treated grape fruits. Ultrason. Sonochem. 2014, 21, 729–734. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals—Promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2006, 120, 451–458. [Google Scholar] [CrossRef]
- Dobson, G.; McDougall, G.J.; Stewart, D.; Cubero, M.Á.; Karjalainen, R.O. Effects of Juice Matrix and Pasteurization on Stability of Black Currant Anthocyanins during Storage. J. Food Sci. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019, 58, 104609. [Google Scholar] [CrossRef]
- Khandpur, P.; Gogate, P.R. Understanding the effect of novel approaches based on ultrasound on sensory profile of orange juice. Ultrason. Sonochem. 2015, 27, 87–95. [Google Scholar] [CrossRef]
- Liao, H.; Jiang, L.; Cheng, Y.; Liao, X.; Zhang, R. Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice. Ultrason. Sonochem. 2018, 42, 244–249. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Martínez-Girón, J.; Arias-Jaramillo, M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017, 233, 96–100. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef] [Green Version]
- Aadil, R.M.; Zeng, X.-A.; Sun, D.-W.; Wang, M.-S.; Liu, Z.; Zhang, Z.-H.; Liu, Z.W. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT-Food Sci. Technol 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Kahraman, O.; Lee, H.; Zhang, W.; Feng, H. Manothermosonication (MTS) treatment of apple-carrot juice blend for inactivation of Escherichia coli 0157:H7. Ultrason. Sonochem. 2017, 38, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Martínez, I.; Mulet, A.; García-Alvarado, M.; Benedito, J. Orange juice processing using a continuous flow ultrasound-assisted supercritical CO 2 system: Microbiota inactivation and product quality. Innov. Food Sci. Emerg. 2018, 47, 362–370. [Google Scholar] [CrossRef]
- Ferrario, M.; Alzamora, S.M.; Guerrero, S. Study of the inactivation of spoilage microorganisms in apple juice by pulsed light and ultrasound. Food Microbiol. 2015, 46, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, A.E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Cruz-Cansino, N.D.S.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; Manríquez-Torres, J.D.J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochem. 2015, 27, 277–286. [Google Scholar] [CrossRef]
- Gomes, W.F.; Tiwari, B.K.; Rodriguez, Ó.; De Brito, E.S.; Fernandes, F.A.N.; Rodrigues, S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chem. 2017, 218, 261–268. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Park, J.-S.; Ha, J.-W. Ultrasound treatment combined with fumaric acid for inactivating food-borne pathogens in apple juice and its mechanisms. Food Microbiol. 2019, 84, 103277. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-López, A.; Martín-Belloso, O. Use of nisin and other bacteriocins for preservation of dairy products. Int. Dairy J. 2008, 18, 329–343. [Google Scholar] [CrossRef]
- Cao-Hoang, L.; Grégoire, L.; Chaine, A.; Waché, Y. Importance and efficiency of in-depth antimicrobial activity for the control of listeria development with nisin-incorporated sodium caseinate films. Food Control. 2010, 21, 1227–1233. [Google Scholar] [CrossRef]
- Rojo, M.C.; Cristiani, M.; Szerman, N.; Gonzalez, M.L.; Lerena, M.C.; Mercado, L.A.; Combina, M. Reduction of Zygosaccharomyces rouxii Population in Concentrated Grape Juices by Thermal Pasteurization and Hydrostatic High Pressure Processing. Food Bioprocess Technol. 2019, 12, 781–788. [Google Scholar] [CrossRef]
- Cao, X.; Cai, C.-F.; Wang, Y.; Zheng, X. The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 169–178. [Google Scholar] [CrossRef]
- Marszałek, K.; Kruszewski, B.; Woźniak, Ł.; Skąpska, S. The application of supercritical carbon dioxide for the stabilization of native and commercial polyphenol oxidases and peroxidases in cloudy apple juice (cv. Golden Delicious). Innov. Food Sci. Emerg. Technol. 2017, 39, 42–48. [Google Scholar] [CrossRef]
- Ma, T.; Lan, T.; Ju, Y.; Cheng, G.; Que, Z.; Geng, T.; Fang, Y.-L.; Sun, X. Comparison of the nutritional properties and biological activities of kiwifruit (Actinidia) and their different forms of products: Towards making kiwifruit more nutritious and functional. Food Funct. 2019, 10, 1317–1329. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, X.; Zhang, J.; Ju, Y.; Que, Z.; Liao, X.; Lao, F.; Fang, Y.; Ma, T.; Cheng, G. Letting wine polyphenols functional: Estimation of wine polyphenols bioaccessibility under different drinking amount and drinking patterns. Food Res. Int. 2020, 127, 108704. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative Analysis of Physicochemical Characteristics, Nutritional and Functional Components and Antioxidant Capacity of Fifteen Kiwifruit (Actinidia) Cultivars—Comparative Analysis of Fifteen Kiwifruit (Actinidia) Cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Deng, W.; Cai, J.; Chen, J. Evaluation of aroma characteristics of sugarcane (Saccharum officinarum L.) juice using gas chromatography-mass spectrometry and electronic nose. LWT 2019, 108, 400–406. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, H.; Lan, T.; Liu, R.; Gao, T.; Yang, W.; Zhou, Y.; Ge, Q.; Fang, Y.; et al. Is overnight fresh juice drinkable? The shelf life prediction of non-industrial fresh watermelon juice based on the nutritional quality, microbial safety quality, and sensory quality. Food Nutr. Res. 2020, 64, 4237. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. A comparative study on the effect of conventional thermal pasteurisation, microwave and ultrasound treatments on the antioxidant activity of five fruit juices. Food Sci. Technol. Int. 2015, 22, 288–301. [Google Scholar] [CrossRef] [PubMed]
- De Arauz, L.J.; Jozala, A.F.; Mazzola, P.G.; Penna, T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Mason, T.J. Some neglected or rejected paths in sonochemistry—A very personal view. Ultrason. Sonochem. 2015, 25, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT 2019, 107, 299–307. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ye, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Atalar, I.; Sarıcaoglu, F.T.; Odabaş, H.I.; Yilmaz, V.A.; Gul, O.; Yılmaz, V.A. Effect of ultrasonication treatment on structural, physicochemical and bioactive properties of pasteurized rosehip (Rosa canina L.) nectar. LWT 2020, 118, 108850. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, L.; Cao, L.; Lin, W.; Ye, X. Sonication inhibited browning but decreased polyphenols contents and antioxidant activity of fresh apple (Malus pumila mill, cv. Red Fuji) juice. J. Food Sci. Technol. 2015, 52, 8336–8342. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wang, Y.; Hu, X.; Wu, J.; Liao, X. Effect of high pressure carbon dioxide on the quality of carrot juice. Innov. Food Sci. Emerg. Technol. 2009, 10, 321–327. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Coupland, J.N.; Anantheswaran, R.C. Characterization of nisin containing chitosan-alginate microparticles. Food Hydrocoll. 2017, 69, 301–307. [Google Scholar] [CrossRef]
- Turfan, Ö.; Türkyılmaz, M.; Yemiş, O.; Özkan, M. Anthocyanin and colour changes during processing of pomegranate (Punica granatum L., cv. Hicaznar) juice from sacs and whole fruit. Food Chem. 2011, 129, 1644–1651. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef]
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Apichartsrangkoon, A.; Wongfhun, P.; Gordon, M.H. Flavor Characterization of Sugar-Added Pennywort (Centella asiatica L.) Juices Treated with Ultra-High Pressure and Thermal Processes. J. Food Sci. 2009, 74, C643–C646. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhan, P.; Tian, H.; Zhang, F.; Xi, J. Characterization of the Influence of Thermal Sterilization on the Volatiles in Flat Peach Juice. Anal. Lett. 2018, 51, 2340–2350. [Google Scholar] [CrossRef]
- Vorobioff, J.; Videla, E.; Boggio, N.; Salomón, O.; Lamagna, A.; Rinaldi, C. Laser Vaporization e-Nose method for the detection of transmitter of Chagas disease. Sens. Actuat. B Chem. 2018, 257, 200–206. [Google Scholar] [CrossRef]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva di Troia grapes by aromatic series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.-L.; Martínez, M.; Simal-Gandara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Filho, E.G.A.; Silva, L.M.A.; De Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.; Rabelo, M.C.; Fonteles, T.V.; Rodrigues, S. Evaluation of thermal and non-thermal processing effect on non-prebiotic and prebiotic acerola juices using 1 H q NMR and GC–MS coupled to chemometrics. Food Chem. 2018, 265, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Fan, X. Measurement of malonaldehyde in apple juice using GC–MS and a comparison to the thiobarbituric acid assay. Food Chem. 2002, 77, 353–359. [Google Scholar] [CrossRef]








| Processing Method | Color | |||
|---|---|---|---|---|
| L* | a* | b* | ΔE | |
| Control group | 56.67 ± 2.32 a | 15.05 ± 0.30 a | 26.31 ± 0.23 a | 0.00 ± 0.00 d |
| CTS | 56.13 ± 0.70 a | 7.50 ± 0.52 c | 5.12 ± 0.85 d | 22.51 ± 0.99 a |
| TS | 48.30 ± 0.62 c | 10.60 ± 0.18 b | 15.32 ± 0.59 c | 14.53 ± 0.06 b |
| TSN | 51.42 ± 0.43 b | 11.09 ± 0.11 b | 17.19 ± 0.97 b | 11.24 ± 0.95 c |
| NO | Ret. Time | Concentration (µg/L) | CAS | RI | ||||
|---|---|---|---|---|---|---|---|---|
| Compounds | Control Group | CTS | TS | TSN | ||||
| 1 | 4.293 | (S)-l-Alanine ethylamide | 2.65 ± 0.096 a | 1.91 ± 0.062 c | 2.27 ± 0.043 b | 2.69 ± 0.066 a | 71773-95-0 | 1097 |
| 2 | 4.517 | Acetaldehyde | 1.27 ± 0.087 bc | 1.64 ± 0.080 a | 1.22 ± 0.091 c | 1.40 ± 0.020 b | 75-07-0 | 408 |
| 3 | 4.79 | Ethanol | 105.52 ± 7.113 a | 89.10 ± 5.007 b | 91.46 ± 5.047 b | 98.94 ± 2.419 ab | 64-17-5 | 463 |
| 4 | 4.997 | Acetone | - | 2.26 ± 0.090 a | - | - | 67-64-1 | 455 |
| 5 | 5.063 | Isopropyl Alcohol | 5.67 ± 0.372 a | 4.95 ± 0.100 ab | 4.53 ± 0.708 b | 4.92 ± 0.426 ab | 67-63-0 | 482 |
| 6 | 5.343 | Acetic acid, methyl ester | - | - | 1.93 ± 0.025 a | 2.06 ± 0.100 a | 79-20-9 | 487 |
| 7 | 6.52 | 3-methyl-4-oxo-Pentanoic acid | 733.41 ± 23.161 a | 654.13 ± 11.903 b | 615.57 ± 6.538 c | 672.90 ± 11.100 b | 6628-79-1 | 1046 |
| 8 | 7.57 | Isopropyl acetate | 6.29 ± 0.780 a | 5.77 ± 0.361 ab | 4.71 ± 0.181 c | 5.39 ± 0.180 bc | 108-21-4 | 621 |
| 9 | 8.637 | Triethylamine | - | - | - | 2.36 ± 0.265 a | 121-44-8 | 667 |
| 10 | 8.98 | n-Propyl acetate | 3.55 ± 0.108 a | 2.98 ± 0.035 b | 2.73 ± 0.119 c | 2.85 ± 0.040 bc | 109-60-4 | 686 |
| 11 | 10.64 | Propanoic acid 2-methyl-ethyl ester | 1.42 ± 0.036 a | 1.13 ± 0.010 b | 0.96 ± 0.015 c | 0.98 ± 0.026 c | 97-62-1 | 721 |
| 12 | 11.89 | Hexanal | 67.91 ± 0.392 a | 64.81 ± 0.934 b | 50.46 ± 0.123 d | 54.36 ± 0.672 c | 66-25-1 | 806 |
| 13 | 12.193 | Butanoic acid ethyl ester | 24.14 ± 1.125 a | 20.99 ± 0.066 b | 16.63 ± 0.203 d | 18.51 ± 0.050 c | 105-54-4 | 785 |
| 14 | 13.683 | 2-Hexenal | - | 1.96 ± 0.061 a | - | - | 505-57-7 | 814 |
| 15 | 13.84 | (E)-2-Butenoic acid ethyl ester | 12.17 ± 0.686 a | 10.11 ± 0.290 b | 8.42 ± 0.271 c | 8.15 ± 0.015 c | 623-70-1 | 793 |
| 16 | 13.997 | (E)-2-Hexenal | 170.46 ± 8.243 a | 148.82 ± 1.460 b | 114.75 ± 0.051 d | 132.93 ± 2.033 c | 6728-26-3 | 814 |
| 17 | 14.397 | Butanoic acid 2-methyl-ethyl ester | 2.06 ± 0.162 a | 1.70 ± 0.017 b | 1.34 ± 0.111 c | 1.48 ± 0.010 c | 7452-79-1 | 820 |
| 18 | 14.517 | 3-Hexen-1-ol | - | 1.91 ± 0.107 b | 1.96 ± 0.030 b | 2.09 ± 0.067 a | 544-12-7 | 868 |
| 19 | 14.523 | (Z)-3-Hexen-1-ol | 2.22 ± 0.127 a | - | 1.97 ± 0.032 b | 1.98 ± 0.015 b | 928-96-1 | 868 |
| 20 | 14.997 | (E)-2-Hexen-1-ol | 3.64 ± 0.136 a | - | 2.36 ± 0.059 b | - | 928-95-0 | 868 |
| 21 | 15.003 | (Z)-2-Hexen-1-ol | - | 2.49 ± 0.083 b | - | 2.69 ± 0.044 a | 928-94-9 | 868 |
| 22 | 15.14 | 1-Hexanol | 9.25 ± 0.127 a | 6.48 ± 0.134 c | - | 8.58 ± 0.096 b | 111-27-3 | 860 |
| 23 | 16.853 | methoxy-phenyl-Oxime | 4.83 ± 0.396 bc | 5.10 ± 0.256 ab | 4.56 ± 0.030 c | 5.40 ± 0.021 a | 0-00-0 | 1301 |
| 24 | 20.427 | 2-Octanone | 2.58 ± 0.015 a | 2.39 ± 0.026 b | 2.44 ± 0.087 b | 2.18 ± 0.042 c | 111-13-7 | 952 |
| 25 | 20.947 | Hexanoic acid ethyl ester | 10.0 ± 0.059 a | 9.23 ± 0.222 b | 5.95 ± 0.100 d | 6.48 ± 0.177 c | 123-66-0 | 984 |
| 26 | 21.65 | 2,2-dimethyl-Decane | - | 3.21 ± 0.272 a | 2.71 ± 0.021 b | 1.63 ± 0.036 c | 17302-37-3 | 1130 |
| 27 | 22.28 | 1-methyl-4-(methylethylidene)-cyclohexene | - | 1.35 ± 0.010 a | - | - | 586-62-9 | 1052 |
| 28 | 22.4 | o-Cymene | 2.26 ± 0.256 b | 2.98 ± 0.135 a | 2.06 ± 0.095 b | 2.04 ± 0.025 b | 527-84-4 | 1042 |
| 29 | 22.833 | D-Limonene | 7.28 ± 0.095 a | 7.92 ± 0.081 a | 7.82 ± 0.749 a | 7.42 ± 0.252 a | 5989-27-5 | 1018 |
| 30 | 23.08 | Bis(2-chloro-1-methylethyl) ether | 3.22 ± 0.079 a | 2.66 ± 0.082 b | 2.09 ± 0.015 c | 2.20 ± 0.165 c | 108-60-1 | 1016 |
| 31 | 23.193 | 1,1’-oxybis 3-chloro-Propane | 1.15 ± 0.006 a | 0.94 ± 0.015 b | 0.77 ± 0.117 c | 0.92 ± 0.032 b | 629-36-7 | 1144 |
| 32 | 24.013 | gamma-Terpinene | 1.20 ± 0.049 b | 1.68 ± 0.031 a | 0.82 ± 0.046 c | 0.75 ± 0.076 c | 99-85-4 | 998 |
| 33 | 24.433 | 3,7-dimethyl-Decane | 0.57 ± 0.006 a | 0.55 ± 0.072 ab | 0.48 ± 0.032 b | 0.56 ± 0.010 a | 17312-54-8 | 1086 |
| 34 | 29.1 | Decanal | - | 1.51 ± 0.378 a | - | 0.97 ± 0.087 b | 112-31-2 | 1204 |
| 35 | 37.703 | 3,5-bis(1,1-dimethylethyl)-Phenol | 6.54 ± 0.411 b | 8.89 ± 0.092 a | - | - | 1138-52-9 | 1555 |
| 36 | 37.71 | 2,4-Di-tert-butylphenol | - | - | 9.29 ± 0.240 a | 7.33 ± 0.270 b | 96-76-4 | 1555 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Wang, J.; Wang, L.; Yang, Y.; Yang, W.; Wang, H.; Lan, T.; Zhang, Q.; Sun, X. Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality. Foods 2020, 9, 1512. https://doi.org/10.3390/foods9101512
Ma T, Wang J, Wang L, Yang Y, Yang W, Wang H, Lan T, Zhang Q, Sun X. Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality. Foods. 2020; 9(10):1512. https://doi.org/10.3390/foods9101512
Chicago/Turabian StyleMa, Tingting, Jiaqi Wang, Lukai Wang, Yanhao Yang, Wanyi Yang, Haoli Wang, Tian Lan, Qianwen Zhang, and Xiangyu Sun. 2020. "Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality" Foods 9, no. 10: 1512. https://doi.org/10.3390/foods9101512
APA StyleMa, T., Wang, J., Wang, L., Yang, Y., Yang, W., Wang, H., Lan, T., Zhang, Q., & Sun, X. (2020). Ultrasound-Combined Sterilization Technology: An Effective Sterilization Technique Ensuring the Microbial Safety of Grape Juice and Significantly Improving Its Quality. Foods, 9(10), 1512. https://doi.org/10.3390/foods9101512