Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw
Abstract
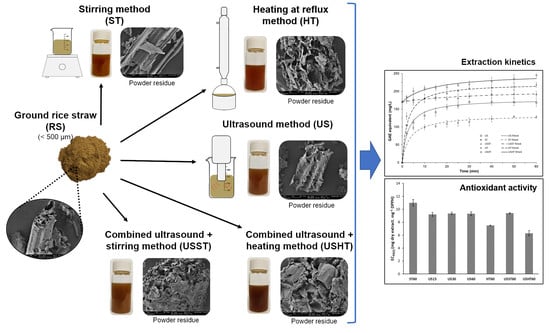
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Preparation
2.3. Aqueous Extraction of RS Powder
2.4. Analysis of Total Phenolic Content
2.5. Modelling Extraction Kinetics
2.6. Antioxidant Activity by DPPH Radical Scavenging Method
2.7. Antibacterial Bioactivity
2.8. Particle Size Distribution in RS Powder
2.9. High-Resolution Field Emission Scanning Electron Microscopy (FESEM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Kinetics of Phenolic Compounds
3.2. Extraction Kinetics Modeling
3.3. Changes in the Plant Tissue Produced by Different Extraction Processes
3.4. Bioactive Characterization of the Extracts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, B.; Vaish, B.; Monika; Singh, U.K.; Singh, P.; Singh, R.P. Recycling of Organic Wastes in Agriculture: An Environmental Perspective. Int. J. Environ. Res. 2019, 13, 409–429. [Google Scholar] [CrossRef]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B: Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 4 November 2020).
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Takano, M.; Hoshino, K. Bioethanol production from rice straw by simultaneous saccharification and fermentation with statistical optimized cellulase cocktail and fermenting fungus. Bioresour. Bioprocess 2018, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Krishania, M.; Kumar, V.; Sangwan, R.S. Integrated approach for extraction of xylose, cellulose, lignin and silica from rice straw. Bioresour. Technol. Rep. 2018, 1, 89–93. [Google Scholar] [CrossRef]
- Jani, S.M.; Rushdan, I. Mechanical properties of beating pulp and paper from rice straw. J. Trop. Agric. Food Sci. 2016, 44, 103–109. [Google Scholar]
- Elhussieny, A.; Faisal, M.; D’Angelo, G.; Aboulkhair, N.T.; Everitt, N.M.; Fahim, I.S. Valorisation of shrimp and rice straw waste into food packaging applications. Ain Shams Eng. J. 2020, S2090447920300101. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of natural antioxidants from rice straw into renewable starch films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Li, Y.; Qi, B.; Luo, J.; Khan, R.; Wan, Y. Separation and concentration of hydroxycinnamic acids in alkaline hydrolyzate from rice straw by nanofiltration. Sep. Purif. Technol. 2015, 149, 315–321. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crop. Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.-C.; Wu, J.-Y. Kinetic models and process parameters for ultrasound-assisted extraction of water-soluble components and polysaccharides from a medicinal fungus. Biochem. Eng. J. 2013, 79, 214–220. [Google Scholar] [CrossRef]
- Ojha, K.S.; Aznar, R.; O’Donnell, C.; Tiwari, B.K. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. TRAC Trends Anal. Chem. 2020, 122, 115663. [Google Scholar] [CrossRef]
- Luque-Garcı́a, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. TRAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Ismail, B.B.; Guo, M.; Pu, Y.; Wang, W.; Ye, X.; Liu, D. Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrason. Sonochem. 2019, 52, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Selectivity of ultrasound-assisted aqueous extraction of valuable compounds from flesh and peel of apple tissues. LWT 2018, 93, 511–516. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Karimi, E.; Mehrabanjoubani, P.; Keshavarzian, M.; Oskoueian, E.; Jaafar, H.Z.; Abdolzadeh, A. Identification and quantification of phenolic and flavonoid components in straw and seed husk of some rice varieties (Oryza sativa L.) and their antioxidant properties: Identification and quantification of phenolic and flavonoid. J. Sci. Food Agric. 2014, 94, 2324–2330. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Abdi, R.D.; Kerro Dego, O. Antimicrobial activity of Persicaria pensylvanica extract against Staphylococcus aureus. Eur. J. Integr. Med. 2019, 29, 100921. [Google Scholar] [CrossRef]
- Requena, R.; Jiménez-Quero, A.; Vargas, M.; Moriana, R.; Chiralt, A.; Vilaplana, F. Integral Fractionation of Rice Husks into Bioactive Arabinoxylans, Cellulose Nanocrystals, and Silica Particles. ACS Sustain. Chem. Eng. 2019, 7, 6275–6286. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Liu, X.; Zhang, X.; Xu, Y.; Leng, F.; Avwenagbiku, M.O. Kinetic modeling of the ultrasonic-assisted extraction of polysaccharide from Nostoc commune and physicochemical properties analysis. Int. J. Biol. Macromol. 2019, 128, 421–428. [Google Scholar] [CrossRef]
- González, N.; Elissetche, J.; Pereira, M.; Fernández, K. Extraction of polyphenols from and: Experimental kinetics, modeling and evaluation of their antioxidant and antifungical activities. Ind. Crop. Prod. 2017, 109, 737–745. [Google Scholar] [CrossRef]
- Dutta, R.; Sarkar, U.; Mukherjee, A. Pseudo-kinetics of batch extraction of Crotalaria juncea (Sunn hemp) seed oil using 2-propanol. Ind. Crop. Prod. 2016, 87, 9–13. [Google Scholar] [CrossRef]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Hayat, K.; Abbas, S.; Hussain, S.; Shahzad, S.A.; Tahir, M.U. Effect of microwave and conventional oven heating on phenolic constituents, fatty acids, minerals and antioxidant potential of fennel seed. Ind. Crop. Prod. 2019, 140, 111610. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef]
- Purohit, A.J.; Gogate, P.R. Ultrasound-Assisted Extraction of β-Carotene from Waste Carrot Residue: Effect of Operating Parameters and Type of Ultrasonic Irradiation. Sep. Sci. Technol. 2015, 50, 1507–1517. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Miyachi, Y. Antioxidant action of natural health products and Chinese herbs. Inflammation 1986, 10, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Faccio, R.; Pistón, M. Characterization of the effects involved in ultrasound-assisted extraction of trace elements from artichoke leaves and soybean seeds. Ultrason. Sonochem. 2019, 59, 104752. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-J.; Sakoda, A. Assessment of the structural factors controlling the enzymatic saccharification of rice straw cellulose. Biomass Bioenergy 2014, 71, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of isoflavones from soy beverages blended with fruit juices. Anal. Chim. Acta 2007, 597, 265–272. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Wei, H.; Hu, J.; Gao, M.-T. Structure analysis of condensed tannin from rice straw and its inhibitory effect on Staphylococcus aureus. Ind. Crop. Prod. 2020, 145, 112130. [Google Scholar] [CrossRef]




| Extraction Method 1 | k (× 10−3) (L·mg−1·min−1) | Ce (mg·L−1) | h (mg·L−1·min−1) | R2 | AARD% |
|---|---|---|---|---|---|
| US | 2.12 | 178.6 | 67.60 | 0.998 | 4.89 |
| ST | 2.24 | 133.3 | 39.82 | 0.994 | 7.76 |
| HT | 1.91 | 222.2 | 94.32 | 0.992 | 4.08 |
| USST | 0.84 | 205.3 | - | 0.980 | 1.14 |
| USHT | 0.93 | 250.3 | - | 0.985 | 2.14 |
| Method 1 | TSY (g Dry Extract. 100 g−1 RS) * | TPC1 (mg GAE 100−1 g RS) * | TPC2 (mg GAE. g−1 Dry Extract) * | EC50(1) (g RS. mg−1 DPPH) * | EC50(2) (mg Dry Extract. mg−1 DPPH) * |
|---|---|---|---|---|---|
| ST60 | 5.61 ± 0.07 a | 256 ± 3 a | 45.7 ± 0.1 a,b | 19.7 ± 0.6 a | 11.0 ± 0.5 a |
| US15 | 5.7 ± 0.3 a | 284 ± 17 b | 49.2 ± 0.9 a | 16.0 ± 1.3 b | 9.2 ± 0.3 b |
| US30 | 7.48 ± 0.05 b | 342 ± 10 c | 45.7 ± 1.0 a,b | 12.5 ± 0.1 c | 9.35 ± 0.18 b |
| US60 | 9.48 ± 0.17 c | 354 ± 12 c | 37.4 ± 1.9 c,d | 9.8 ± 0.3 d | 9.3 ± 0.3 b |
| HT60 | 9.6 ± 0.6 c | 459 ± 6 d | 47.0 ± 3.0 a | 7.8 ± 0.5 e | 7.49 ± 0.05 c |
| USST60 | 9.0 ± 0.4 c | 382 ± 6 e | 42.3 ± 2.4 b,c | 10.4 ± 0.3 f | 9.39 ± 0.10 b |
| USHT60 | 13.95 ± 0.13 d | 486 ± 4 f | 34.8 ± 0.5 d | 4.6 ± 0.3 g | 6.3 ± 0.4 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw. Foods 2020, 9, 1657. https://doi.org/10.3390/foods9111657
Freitas PAV, González-Martínez C, Chiralt A. Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw. Foods. 2020; 9(11):1657. https://doi.org/10.3390/foods9111657
Chicago/Turabian StyleFreitas, Pedro A. V., Chelo González-Martínez, and Amparo Chiralt. 2020. "Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw" Foods 9, no. 11: 1657. https://doi.org/10.3390/foods9111657
APA StyleFreitas, P. A. V., González-Martínez, C., & Chiralt, A. (2020). Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw. Foods, 9(11), 1657. https://doi.org/10.3390/foods9111657