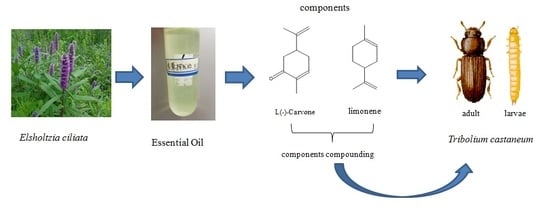
Toxicity and Synergistic Effect of Elsholtzia ciliata Essential Oil and Its Main Components against the Adult and Larval Stages of Tribolium castaneum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction of Essential Oil
2.2. Test Insects
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Contact Toxicity
2.5. Fumigant Toxicity
2.6. Two Main Components Compounding
2.7. Data Analysis
- ①
- Co-toxicity index (CTC) = ATI/TTI × 100%
- ②
- Mixed virulence index (ATI) = standard drug LD50/mixture (A+B) LD50 × 100%
- ③
- Theoretical virulence index of (A+B) (TTI) = Va × Ma + Vb × MbVa = Virulence index of agent A, Ma = the mass fraction of agent A in the mixtureVb = Virulence index of agent B, Mb = the mass fraction of agent B in the mixture
- ④
- Single dose virulence index (TI) = standard drug LD50/LD50 for the test agent × 100%
2.8. Chemicals
3. Results
3.1. Chemical Compounds of E. ciliata Essential Oil
3.2. Contact Activity
3.3. Fumigation Activity
3.4. Carvone Mixed with Limonene and Its Contact Toxicity against T. castaneum Adult
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Athanassiou, C.G.; Arthur, F.H.; Throne, J.E. Efficacy of grain protectants against four psocid species on maize, rice and wheat. Pest Manag. 2009, 65, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Tapondjou, A.L.; Alder, C.; Bouda, H.; Fontem, D.A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.F.; Yang, K.; You, C.X.; Zhang, W.J.; Guo, S.S.; Geng, Z.F.; Du, S.S.; Wang, Y.Y. Chemical composition and insecticidal activity of essential oils from Zanthoxylum dissitum leaves and roots against three species of storage pests. Molecules 2015, 20, 7990–7999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.F.; Chen, H.J.; Xue, G.H. Atlas of Beetles Associated with Stored Products; China Agricultural Science and Technology Press: Beijing, China, 2008; p. 161. ISBN 978-78-0233-607-0. [Google Scholar]
- Ebadollahi, A.; Safaralizadeh, M.H.; Pourmirza, A.A.; Gheibi, S.A. Toxicity of essential oil of Agastache foeniculum (Pursh) kuntze to Oryzaephilus surinamensis L. and Lasioderma serricorne F. Plant Prot. Res. 2010, 50, 215–219. [Google Scholar] [CrossRef]
- Isman, M.B.; Singh, B.P. Botanical insecticides, deterrents, repellents and oils. Ind. Crops Uses 2010, 20, 433–445. [Google Scholar] [CrossRef]
- Zettler, J.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crops Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Fabres, A.; Janaina, C.M.D.S.; Fernandes, K.V.S.; Xavier-Filho, J.; Rezende, G.L.; Oliveira, A.E.A. Comparative performance of the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae) on different plant diets. J. Pestic. Sci. 2014, 87, 495–506. [Google Scholar] [CrossRef]
- Bossou, A.D.; Ahoussi, E.; Ruysbergh, E.; Adams, A.; Smagghe, G.D.; Kimpe, N.; Mangelinckx, S. Characterization of volatile compounds from three Cymbopogon species and Eucalyptus citriodora from benin and their insecticidal activities against Tribolium castaneum. Ind. Crops Prod. 2015, 76, 306–317. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, H.S. Toxicities of active constituent isolated from Thymus vulgaris flowers and its structural derivatives against Tribolium castaneum (Herbst). Appl. Biol. Chem. 2016, 59, 821–826. [Google Scholar] [CrossRef]
- Regnaltroger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low–risk products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Chu, S.S.; Jiang, G.H.; Liu, W.L.; Liu, Z.L. Insecticidal activity of the root bark essential oil of Periploca sepium Bunge and its main component. Nat. Prod. Res. 2012, 26, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Lalonde, M.; Marcotte, A. Insect growth-reducing and antifeedant activity in Eastern North America hardwood species and bioassay-guided isolation of active principles from Prunus serotina. Agric. For. Entomol. 2000, 2, 253–257. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abd-Alla, A.M.; Moharramipour, S. Combination of gamma radiation and essential oils from medicinal plants in managing Tribolium castaneum contamination of stored products. Appl. Radiat. Isot. 2013, 78, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Zhou, L.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Evaluation of the toxicity of the essential oils of some common Chinese spices agaist Liposcelis bostrychophila. Food Control 2012, 26, 486–490. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep. 2015, 5, 12690. [Google Scholar] [CrossRef] [Green Version]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2019, 1–7. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Metabolism of citral, the major constituent of lemongrass oil, in the cabbage looper, Trichoplusia ni, and effects of enzyme inhibitors on toxicity and metabolism. Pestic. Biochem. Phys. 2016, 133, 20–25. [Google Scholar] [CrossRef]
- Tak, J.H.; Jovel, E.; Isman, M.B. Effects of rosemary, thyme and lemongrass oils and their major constituents on detoxifying enzyme activity and insecticidal activity in Trichoplusia ni. Pestic. Biochem. Phys. 2017, 140, 9–16. [Google Scholar] [CrossRef]
- Aref, S.P.; Valizadegan, O.; Farashiani, M.E. The Insecticidal Effect of Essential Oil of Eucalyptus floribundi Against Two Major Stored Product Insect Pests; Rhyzopertha dominica (F.) and Oryzaephilus surinamensis (L.). J. Plant Prot. Res. 2015, 55, 35–41. [Google Scholar] [CrossRef]
- Du, X.W.; Tan, J.C.; Cao, Y.F.; Zhang, X.H.; Chen, J.S. Research progress in plant essential oils. Hunan Agric. Sci. 2009, 8, 86–89. [Google Scholar] [CrossRef]
- Neffati, A.; Bouhlel, I.; Sghaier, M.B.; Boubaker, J.; Limen, I.; Kilani, S.; Skandrani, I.; Bhouri, W.; Dauphin, J.L.; Barillier, D.; et al. Antigenotoxic and antioxidant activities of Pituranthos chloranthus essential oils. Environ. Toxicol. Pharmacl. 2009, 27, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.G.; Luo, M.T.; Wei, H. Study on the antibacterial effect of 8 kinds of plant essential oils on common intestinal microorganisms in vitro. Mod. Food Sci. Technol. 2017, 6, 133–141. [Google Scholar]
- Oke, F.; Aslim, B.; Ozturk, S.; Altundag, S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009, 112, 874–879. [Google Scholar] [CrossRef]
- Li, H.X.; Wei, M.S.; Yi, P.Y.; Ke, Z.G.; Man, Y.S. The control effect of 25 kinds of plant essential oils on the Callosobruchus maculatus. Grain Storage 2001, 30, 7–9. [Google Scholar]
- Zhao, M.P.; Liu, X.C.; Lai, D.W.; Zhao, L.; Liu, Z.L. Analysis of the Essential Oil of Elsholtzia ciliata Aerial Parts and Its Insecticidal Activities against Liposcelis bostrychophila. Helv. Chim. Acta 2016, 99, 90–94. [Google Scholar] [CrossRef]
- Lv, J.H.; Wu, S.H.; Yuan, L.Y.; Li, Y.F. Study on the effect of Elsholtzia stauntonii benth extract on the adults of Sitophilus zeamais and Tribolium rubra. J. Henan Univ. Technol. Nat. Sci. E 2008, 29, 31–34. [Google Scholar]
- De Carvalho, C.C.; Da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Ma, C.; Liu, X.L.; Zhao, Z.G.; Zhang, L. Improvement on the synthesis of carvone perfume. J. Southwest Univ. Nat. Sci. Ed. 2003, 308–309, 340. [Google Scholar] [CrossRef]
- Committee, E.S. Scientific Opinion on the safety assessment of carvone, considering all sources of exposure. EFSA J. 2014, 12, 1–74. [Google Scholar] [CrossRef]
- Wang, W.J. Recent advances on limonene, a natural and active monoterpene. China Food Addit. 2005, 1, 33–37. [Google Scholar] [CrossRef]
- Sun, J.D. D-limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y. Safety Evaluation And Risk Assessment Of d-Limonene. J. Toxicol. Environ. Health 2013, 16, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jiang, C.H.; Wang, X.Y. Insecticidal Activity of Essential Oil of Carum Carvi Fruits from China and Its Main Components against Two Grain Storage Insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef]
- Yang, B. Study on Fumigation Activity of Plant Essential Oil. D; Huazhong Agriculture University: Wuhan, China, 2008. [Google Scholar] [CrossRef]
- Zhu, X.K.; Guo, S.S.; Zhang, Z. Insecticidal activities of the essential oil from Alpinia zerumbet leaves against Triboliun castaneum in storag. Plant Prot. 2017, 6, 151–155+162. [Google Scholar] [CrossRef]
- Zhao, X.N.; Wang, J.Q. Study on the fumigant virulence of the oriental green pepper oil to the larvae of the Tribolium castaneum. Packag. Eng. 2012, 33, 9–13. [Google Scholar]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. J. Am. Soc. Mass Spectr. 2005, 16, 1902–1903. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wang, C.F.; You, C.X. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia Pac. Entomol. 2014, 17, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; You, C.X.; Tian, Z.F. Insecticidal Activity of essential Oil from Vitex negundo L. var. cannabifolia Leaves on Lasioderma serricorne. Plant Prot. 2016, 42, 97–102. [Google Scholar] [CrossRef]
- Chen, I.; Xu, H.H.; Li, Y.Y.; He, L.F.; Zhao, S.H. Discussion on the method of screening the best effective formula of pesticide compound. Acta Phytophys. Sin. 2000, 27, 349–354. [Google Scholar] [CrossRef]
- Wang, S.X.; Yuan, H.B.; Ma, L.B.; Yang, C.H.; Reng, B.Z. Fumigation effect of plant essential oil mixed with ethyl formate on adults of Sitophilus zeamais. J. Northeast Norm. Univ. Nat. Sci. 2012, 44, 107–111. [Google Scholar]
- Liu, X.P.; Jing, X.M. Study on chemical constituents and biological activities of E.ciliata essential oil. J. Heilongjiang Bayi Agric. Univ. 2018, 30, 1002–2090. [Google Scholar]
- Jin, X.L.; Li, D.H. Component analysis of essential oil from wild E. ciliata fragrans in Changbai Mountain. J. Yanbian Univ. Nat. Sci. Ed. 1996, 1, 32–34. [Google Scholar]
- Li, W.Q.; Jiang, C.H.; Chu, S.S.; Zuo, M.X.; Liu, Z.L. Chemical Composition and Toxicity against Sitophilus zeamais and Tribolium castaneum of the Essential Oil of Murraya exotica Aerial Parts. Molecules 2010, 15, 5831–5839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.X.; Huang, S.S. The biological activity of eugenol on the Tribolium castaneum. J. Chongqing Norm. Univ. Nat. Sci. Ed. 2009, 26, 16–19. [Google Scholar] [CrossRef]
- Lv, J.H.; Wang, X.M.; Bai, X.G.; Lu, Y.J. Control of four plants essential oils against the Tribolium castaneum research. Henan Agric. Sci. 2006, 9, 68–71. [Google Scholar] [CrossRef]
- Huang, Y.P.; Chen, Q.; Chen, M.D. Exploration Experiments on the Effects of Feeding Conditions on the Development and Reproductive Capacity of Tribolium castaneum. Biol. Teach. 2012, 37, 58–59. [Google Scholar] [CrossRef]
- Liang, Y.S. Behavioral responses of the main components of defensive secretions of adult and larvae of the genus Tribolium castaneum. J. Chin. Cereals Oils Assoc. 1995, 4, 18–22. [Google Scholar]
- Chen, H.P.; Yang, K.; You, C.X.; Du, S.S.; Cai, Q.; He, Q.; Geng, Z.F.; Deng, Z.W. Chemical constituents and biological activities against Tribolium castaneum (Herbst) of the essential oil from Citrus wilsonii leaves. J. Serbian Chem. Soc. 2014, 79, 1213–1222. [Google Scholar] [CrossRef]
- Guo, D.L.; Pu, W.; Yan, X.P.; Tao, C. Research progress of modified atmosphere and fumigation of foreign countries—A review of the 8th Int’l Conference on Controlled Atmosphere and Fumigation Conference (CAF 2004). Grain Storage 2004, 32, 44–48. [Google Scholar] [CrossRef]


| Number | Constituent | Retention Time/Min (Rt) | Ri * | Relative Content (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 3.394 | 932 | 0.55 |
| 2 | β-Pinene | 3.812 | 977 | 0.74 |
| 3 | Myrcene | 3.861 | 988 | 1.02 |
| 4 | β-Phellandrene | 3.966 | 1019 | 0.19 |
| 5 | Limonene | 4.464 | 1040 | 22.05 |
| 6 | β-Ocimene | 4.654 | 1061 | 4.08 |
| 7 | Linalool | 5.367 | 1090 | 0.83 |
| 8 | Elsholtzia ketone | 6.726 | 1199 | 1.02 |
| 9 | Carvone | 7.366 | 1216 | 31.63 |
| 10 | Dehydroelsholtzia ketone | 8.104 | 1277 | 14.86 |
| 11 | Cubebene | 9.180 | 1344 | 1.06 |
| 12 | β-Bourbonene | 9.635 | 1379 | 0.44 |
| 13 | β-Caryophyllen | 10.077 | 1414 | 2.92 |
| 14 | α-Caryophyllene | 10.397 | 1450 | 15.47 |
| 15 | (-)-Humulene epoxide II | 10.643 | 1454 | 0.25 |
| 16 | α-Farnesene | 11.965 | 1489 | 0.47 |
| - | Total | - | - | 97.58 |
| Others | 2.42 |
| T. castaneum | Treatment | Ld50 (mg/Adult) | 95% Fl (mg/Adult) | Slope ± Se | p-Value | Chi Square X2 |
|---|---|---|---|---|---|---|
| Adult | Essential oil | 7.79 | 6.96−8.65 | 4.14 ± 0.46 | 0.85 | 16.17 |
| Carvone | 5.08 | 4.19−6.20 | 4.30 ± 0.46 | 0.01 | 44.15 | |
| Limonene | 38.57 | 34.48−43.09 | 3.84 ± 0.42 | 0.55 | 21.54 | |
| Pyrethrin | 0.09 | 0.08−0.11 | 2.48 ± 0.31 | 0.92 | 14.27 | |
| Larva | Essential oil | 24.87 | 19.55−30.69 | 1.69 ± 0.22 | 0.64 | 24.72 |
| Carvone | 33.03 | 26.55−41.26 | 1.86 ± 0.23 | 0.75 | 18.12 | |
| Limonene | 49.68 | 34.10−84.04 | 0.95 ± 0.15 | 0.54 | 26.70 | |
| Pyrethrin | 1.31 | 0.75−2.17 | 0.80 ± 0.10 | 0.82 | 16.72 |
| T. castaneum | Treatment | LC50 ( mg/L Air) | 95% FL (mg/L Air) | Slope ± SE | p-Value | Chi Square X2 |
|---|---|---|---|---|---|---|
| Adult | Essential oil | 11.61 | 9.21−14.01 | 4.39 ± 0.47 | 0.00 | 87.62 |
| Carvone | 4.34 | 3.90−4.84 | 6.27 ± 0.83 | 0.98 | 7.89 | |
| Limonene | 5.52 | 2.75−9.22 | 1.69 ± 0.47 | 0.83 | 5.85 | |
| Methyl bromide a | 1.83 | 1.43−2.23 | 4.90 ± 0.51 | 0.89 | 8.67 | |
| Larva | Essential oil | 8.73 | 6.62−11.25 | 1.42 ± 0.17 | 0.99 | 11.19 |
| Carvone | 28.71 | 23.07−36.05 | 1.63 ± 0.15 | 0.36 | 35.41 | |
| Limonene | 20.64 | 16.96−25.56 | 1.71 ± 0.16 | 0.86 | 24.46 | |
| Phoxim | 1.05 | 1.23–2.08 | 1.65 ± 0.45 | 0.89 | 3.25 |
| Volume Ratio | LD50 (μg/Adult) | Slope ± SE | p-Value | ATI | TTI | CTC |
|---|---|---|---|---|---|---|
| 1:7 | 24.60 | 2.935 ± 0.59 | 0.64 | 20.65 | 24.02 | 85.97 |
| 2:6 | 10.84 | 2.972 ± 0.51 | 0.54 | 46.85 | 34.88 | 134.33 |
| 3:5 | 34.43 | 1.856 ± 0.45 | 0.99 | 14.75 | 45.73 | 32.26 |
| 4:4 | 39.60 | 1.970 ± 0.47 | 0.99 | 12.83 | 113.17 | 11.33 |
| 5:3 | 140.30 | 1.605 ± 0.79 | 0.60 | 3.62 | 67.43 | 5.37 |
| 6:2 | 79.34 | 1.666 ± 0.95 | 0.95 | 6.40 | 78.29 | 8.18 |
| 7:1 | 434.82 | 1.495 ± 0.85 | 0.80 | 1.17 | 115.93 | 1.01 |
| Volume Ratio | LD50 (μg/Larva) | Slope ± SE | p-Value | ATI | TTI | CTC |
|---|---|---|---|---|---|---|
| 1:7 | 30.04 | 2.323 ± 0.59 | 0.34 | 109.94 | 70.68 | 155.55 |
| 2:6 | 30.62 | 3.829 ± 0.73 | 0.71 | 107.87 | 74.87 | 144.08 |
| 3:5 | 84.30 | 1.145 ± 0.14 | 0.88 | 39.18 | 79.06 | 49.56 |
| 4:4 | 405.96 | 1.390 ± 0.12 | 0.46 | 8.14 | 83.24 | 9.77 |
| 5:3 | 66.30 | 3.074 ± 0.16 | 0.95 | 49.82 | 87.43 | 56.98 |
| 6:2 | 112.98 | 2.175 ± 0.94 | 0.90 | 29.24 | 91.62 | 31.91 |
| 7:1 | 26.48 | 5.321 ± 0.11 | 0.96 | 124.74 | 95.81 | 130.19 |
| Volume Ratio | LC50 (mg/L Air) | Slope ± SE | p-Value | ATI | TTI | CTC |
|---|---|---|---|---|---|---|
| 1:7 | 2.51 | 2.921 ± 0.48 | 0.00 | 172.91 | 81.29 | 212.71 |
| 2:6 | 3.25 | 4.793 ± 0.63 | 0.05 | 133.54 | 83.97 | 159.03 |
| 3:5 | 7.43 | 2.845 ± 0.93 | 0.80 | 58.41 | 86.64 | 67.42 |
| 4:4 | 6.55 | 2.656 ± 0.82 | 0.72 | 66.26 | 89.31 | 74.19 |
| 5:3 | 9.45 | 2.567 ± 0.76 | 0.88 | 45.93 | 91.98 | 49.93 |
| 6:2 | 11.39 | 2.814 ± 0.44 | 0.85 | 38.10 | 94.66 | 40.25 |
| 7:1 | 26.30 | 1.889 ± 0.40 | 0.72 | 16.50 | 97.33 | 16.95 |
| Volume Ratio | LC50 (mg/L Air) | Slope ± SE | p-Value | ATI | TTI | CTC |
|---|---|---|---|---|---|---|
| 1:7 | 38.56 | 1.846 ± 0.70 | 0.87 | 74.46 | 134.21 | 55.48 |
| 2:6 | 34.64 | 2.314 ± 0.77 | 0.80 | 82.88 | 129.32 | 64.09 |
| 3:5 | 31.08 | 2.201 ± 0.71 | 0.88 | 92.37 | 124.44 | 74.23 |
| 4:4 | 32.99 | 2.286 ± 0.72 | 0.94 | 87.03 | 119.55 | 72.79 |
| 5:3 | 27.93 | 3.041 ± 0.79 | 0.54 | 102.79 | 114.66 | 89.65 |
| 6:2 | 221.59 | 1.215 ± 0.85 | 0.81 | 12.96 | 109.76 | 11.80 |
| 7:1 | 52.91 | 3.189 ± 0.46 | 0.88 | 54.26 | 104.89 | 51.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.-Y.; Xu, J.; Yang, Y.-Y.; Shao, Y.-Z.; Zhou, F.; Wang, J.-L. Toxicity and Synergistic Effect of Elsholtzia ciliata Essential Oil and Its Main Components against the Adult and Larval Stages of Tribolium castaneum. Foods 2020, 9, 345. https://doi.org/10.3390/foods9030345
Liang J-Y, Xu J, Yang Y-Y, Shao Y-Z, Zhou F, Wang J-L. Toxicity and Synergistic Effect of Elsholtzia ciliata Essential Oil and Its Main Components against the Adult and Larval Stages of Tribolium castaneum. Foods. 2020; 9(3):345. https://doi.org/10.3390/foods9030345
Chicago/Turabian StyleLiang, Jun-Yu, Jie Xu, Ying-Ying Yang, Ya-Zhou Shao, Feng Zhou, and Jun-Long Wang. 2020. "Toxicity and Synergistic Effect of Elsholtzia ciliata Essential Oil and Its Main Components against the Adult and Larval Stages of Tribolium castaneum" Foods 9, no. 3: 345. https://doi.org/10.3390/foods9030345
APA StyleLiang, J. -Y., Xu, J., Yang, Y. -Y., Shao, Y. -Z., Zhou, F., & Wang, J. -L. (2020). Toxicity and Synergistic Effect of Elsholtzia ciliata Essential Oil and Its Main Components against the Adult and Larval Stages of Tribolium castaneum. Foods, 9(3), 345. https://doi.org/10.3390/foods9030345