Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Solvents
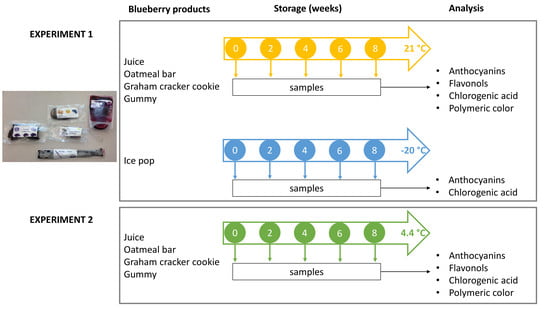
2.2. Preparation of Blueberry Products
2.3. Extraction of Polyphenolics from Freeze-dried Blueberries and Products
2.4. HPLC Analysis of Anthocyanins and Chlorogenic Acid
2.5. HPLC Analysis of Flavonols
2.6. HPLC/ESI-MS Analysis of Polyphenolics
2.7. Analysis of Percent Polymeric Color
2.8. Statistical Analysis
3. Results
3.1. Identification of Anthocyanins by HPLC/MS
3.2. Identification of Flavonols by HPLC/MS
3.3. Stability of Anthocyanins in Blueberry Products during Storage
3.3.1. Ice Pop
3.3.2. Oatmeal Bar
3.3.3. Graham Cracker Cookie
3.3.4. Juice
3.3.5. Gummy Product
3.3.6. Product Comparison of Anthocyanin Composition over Eight Weeks of Storage
3.4. Changes in Percent Polymeric Color in Blueberry Products during Storage
3.4.1. Oatmeal Bar
3.4.2. Graham Cracker Cookie
3.4.3. Juice
3.4.4. Gummy Product
3.5. Stability of Chlorogenic Acid during Storage
3.6. Stability of Flavonols in Blueberry Products during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Böhm, H.G. Mazza und E. Miniati: Anthocyanins in Fruits, Vegetables and Grains. 362 Seiten, zahlr. Abb. und Tab. CRC Press, Boca Raton, Ann Arbor, London, Tokyo 1993. Preis: 144. Food Nahr. 1994, 38, 343. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Ricker, R.D.; Liu, X. Anthocyanin content and profile within and among blueberry species. Can. J. Plant Sci. 1999, 79, 617–623. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Quantitation and Distribution of Simple and Acylated Anthocyanins and Other Phenolics in Blueberries. J. Food Sci. 1994, 59, 1057–1059. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of Procyanidins and Anthocyanins in Blueberries and Cranberries (Vaccinium Spp.) Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E. Chemical Composition of Lowbush Blueberry Cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 142–146. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Palmieri, L.; Mattivi, F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J. Food Compos. Anal. 2012, 25, 9–16. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2019, 11, 224–236. [Google Scholar] [CrossRef]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Zhang, J.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M. Diet-derived phenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Wankhade, U.D.; Alund, A.W.; Blackburn, M.L.; Shankar, K.; Lazarenko, O.P. 3-(3-Hydroxyphenyl)-Propionic Acid (PPA) Suppresses Osteoblastic Cell Senescence to Promote Bone Accretion in Mice. JBMR Plus 2019, 3, e10201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Clark, K.; Schuschke, D.A.; Klimis-Zacas, D.J. Vascular reactivity is affected by dietary consumption of wild blueberries in the Sprague-Dawley rat. J. Med. Food 2009, 12, 21–28. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A study of glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef] [Green Version]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Cifuentes-Gomez, T.; Spencer, J.P.E. Bioavailability of wild blueberry (poly)phenols at different levels of intake. J. Berry Res. 2016, 6, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Whyte, A.R.; Schafer, G.; Williams, C.M. The effect of cognitive demand on performance of an executive function task following wild blueberry supplementation in 7 to 10 years old children. Food Funct. 2017, 8, 4129–4138. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. Characterization of Wild Blueberry Polyphenols Bioavailability and Kinetic Profile in Plasma over 24-h Period in Human Subjects. Mol. Nutr. Food Res. 2017, 61, 1700405. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Adams, S.H.; Howard, L.R.; Brownmiller, C.; Hogan, V.; Chen, J.-R.; Pramudya, R.C. Children’s liking and wanting of foods vary over multiple bites/sips of consumption: A case study of foods containing wild blueberry powder in the amounts targeted to deliver bioactive phytonutrients for children. Food Res. Int. 2020, 131, 108981. [Google Scholar] [CrossRef] [PubMed]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Castrodale, C.; Brownmiller, C.; Mauromoustakos, A. Jam Processing and Storage Effects on Blueberry Polyphenolics and Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in Anthocyanins and Polyphenolics During Juice Processing of Highbush Blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Fracassetti, D.; Del Bo’, C.; Simonetti, P.; Gardana, C.; Klimis-Zacas, D.; Ciappellano, S. Effect of Time and storage temperature on anthocyanin decay and antioxidant activity in wild blueberry (Vaccinium angustifolium) powder. J. Agric. Food Chem. 2013, 61, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Prior, R.L.; Liyanage, R.; Lay, J.O. Processing and Storage Effect on Berry Polyphenols: Challenges and Implications for Bioactive Properties. J. Agric. Food Chem. 2012, 60, 6678–6693. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E. Flavonoids berries and grapes. In Functional Foods: Biochemical and Processing Aspects; CRC Press: Boca Raton, FL, USA, 2012; Volume 2, pp. 71–133. [Google Scholar]
- Howard, L.R.; Brownmiller, C.; Mauromoustakos, A.; Prior, R.L. Improved stability of blueberry juice anthocyanins by acidification and refrigeration. J. Berry Res. 2016, 6, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Simard, R.E.; Bourzeix, M.; Herdia, N. Factors influencing color degradation in blueberry juice. Lebensm. Wiss. Technol. 1982, 15, 177–180. [Google Scholar]
- Zhang, J.; Lazarenko, O.P.; Kang, J.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M.; Chen, J.R. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS ONE 2013, 8, e70438. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 00, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2004, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 2004, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Syamaladevi, R.M.; Andrews, P.K.; Davies, N.M.; Walters, T.; Sablani, S.S. Storage effects on anthocyanins, phenolics and antioxidant activity of thermally processed conventional and organic blueberries. J. Sci. Food Agric. 2012, 92, 916–924. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O.; de Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007, 55, 2705–2713. [Google Scholar] [CrossRef]
- Maier, T.; Fromm, M.; Schieber, A.; Kammerer, D.R.; Carle, R. Process and storage stability of anthocyanins and non-anthocyanin phenolics in pectin and gelatin gels enriched with grape pomace extracts. Eur. Food Res. Technol. Z. Für Lebensm. Unters. Forsch. Heidelb. 2009, 229, 949–960. [Google Scholar] [CrossRef]
- Brouillard, R. Chemical structure of anthocyanins. In Anthocyanins as Food Colors; Academic Press: New York, NY, USA, 1982; pp. 1–40. [Google Scholar]
- Kader, F.; Rovel, B.; Girardin, M.; Metche, M. Mechanism of Browning in Fresh Highbush Blueberry Fruit (Vaccinium corymbosum L). Role of Blueberry Polyphenol Oxidase, Chlorogenic Acid and Anthocyanins. J. Sci. Food Agric. 1997, 74, 31–34. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on blueberry (Vaccinium corymbosum L.) polyphenolics. Acta Hort. 2009, 841, 347–354. [Google Scholar] [CrossRef] [Green Version]






| Peak | HPLC (RT in min) | Identification | m/z [M+] | Fragments |
|---|---|---|---|---|
| 1 | 24.9 | delphinidin-3-galactoside | 465 | 303 |
| 2 | 26.2 | delphinidin-3-glucoside | 465 | 303 |
| 3 | 27.1 | cyanidin-3-galactoside | 449 | 287 |
| 4 | 28.1 | delphinidin-3-arabinoside | 435 | 303 |
| 5 | 28.8 | cyanidin-3-glucoside | 449 | 287 |
| 6 | 29.9 | petunidin-3-galactoside | 479 | 317 |
| 7 | 30.3 | cyanidin-3-arabinoside | 419 | 287 |
| 8 | 31.1 | petunidin-3-glucoside | 479 | 317 |
| 9 | 31.8 | peonidin-3-galactoside | 463 | 301 |
| 10 | 32.9 | petunidin-3-arabinoside | 449 | 317 |
| 11 | 33.2 | peonidin-3-glucoside | 463 | 301 |
| 12 | 33.6 | malvidin-3-galactoside | 493 | 331 |
| 13 | 35.1 | malvidin-3-glucoside | 493 | 331 |
| 14 | 36.6 | malvidin-3-arabinoside | 463 | 331 |
| 15 | 37.7 | cyanidin-3-(6″-malonyl) galactoside + cyanidin-3-(6″-acetyl) galactoside | 535 491 | 287 287 |
| 16 | 40.0 | delphinidin-3-(6″-acetyl) galactoside | 507 | 303 |
| 17 | 41.6 | delphinidin derivative | - | 303 |
| 18 | 42.3 | delphinidin-3-rutinoside malvidin-3-(6″-acetyl) galactoside cyanidin-3-(6″-malonyl) glucoside | 611 535 535 | 303 331 287 |
| 19 | 42.7 | malvidin-3-(6″-acetyl) galactoside | 535 | 331 |
| 20 | 43.6 | petunidin-3-(6″-acetyl) glucoside | 521 | 317 |
| 21 | 45.7 | peonidin-3-(6″-acetyl) glucoside | 505 | 301 |
| 22 | 46.6 | malvidin-3-(6″-acetyl) glucoside | 535 | 331 |
| Peak | HPLC (RT in min) | Identification | m/z [M−] | Fragments |
|---|---|---|---|---|
| 1 | 39.2 | myricetin-3-galactoside | 479 | 316 |
| 2 | 39.8 | myricetin-3-glucoside | 479 | 316 |
| 3 | 43.8 | myricetin-3-rhamnoside | 463 | 316 |
| 4 | 44.2 | quercetin-3-rutinoside | 609 | 300 |
| 5 | 45.0 | quercetin-3-galactoside | 463 | 300 |
| 6 | 45.8 | quercetin-3-glucoside | 463 | 300 |
| 7 | 47.0 | quercetin-3-glucuronide | 477 | 301 |
| 8 | 48.9 | Unknown | 623 | 505, 433, 300 |
| 9 | 50.3 | quercetin-3-pentoside | 433 | 300 |
| 10 | 51.0 | quercetin-3-rhamnoside | 447 | 300 |
| 11 | 51.1 | syringetin-3-galactoside/glucoside | 507 | 344 |
| 12 | 51.8 | quercetin-3-(6″-acetyl) galactoside | 505 | 300 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavefve, L.; Brownmiller, C.; Howard, L.; Reeves, D.; Adams, S.H.; Chen, J.-R.; Diaz, E.C.; Mauromoustakos, A. Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder. Foods 2020, 9, 466. https://doi.org/10.3390/foods9040466
Lavefve L, Brownmiller C, Howard L, Reeves D, Adams SH, Chen J-R, Diaz EC, Mauromoustakos A. Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder. Foods. 2020; 9(4):466. https://doi.org/10.3390/foods9040466
Chicago/Turabian StyleLavefve, Laura, Cindi Brownmiller, Luke Howard, Donovon Reeves, Sean H. Adams, Jin-Ran Chen, Eva C. Diaz, and Andy Mauromoustakos. 2020. "Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder" Foods 9, no. 4: 466. https://doi.org/10.3390/foods9040466
APA StyleLavefve, L., Brownmiller, C., Howard, L., Reeves, D., Adams, S. H., Chen, J. -R., Diaz, E. C., & Mauromoustakos, A. (2020). Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder. Foods, 9(4), 466. https://doi.org/10.3390/foods9040466