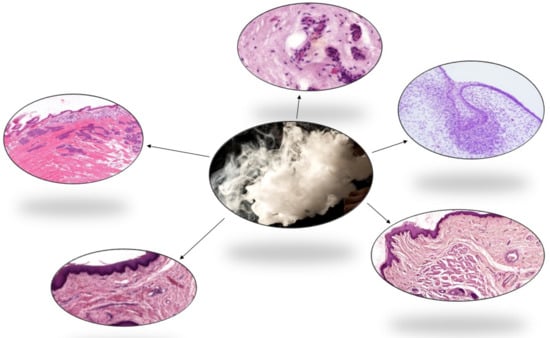
The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review
Abstract
:1. Introduction
2. Chemical Components and Toxins of E-Cigarette Aerosol
3. Effect of E-Cigarettes Aerosol on Oral Cavity
3.1. Oral Microenvironment
3.2. Oral Microbiome
4. Injury of Oral Cavity as Effect of E-Cigarettes Explosion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, D.; Long, J.; Quigley, J.; Lee, C.; McCarthy, A.; Kavanagh, P. Association between electronic cigarette use and tobacco cigarette smoking initiation in adolescents: A systematic review and meta-analysis. BMC Public Health 2021, 21, 954. [Google Scholar] [CrossRef]
- Alolabi, H.; Alchallah, M.O.; Mohsen, F.; Shibani, M.; Ismail, H.; Alzabibi, M.A.; Sawaf, B. Prevalence and behavior regarding cigarette and water pipe smoking among Syrian undergraduates. Heliyon 2020, 6, e05423. [Google Scholar] [CrossRef]
- Adermark, L.; Galanti, M.R.; Ryk, C.; Gilljam, H.; Hedman, L. Prospective association between use of electronic cigarettes and use of conventional cigarettes: A systematic review and meta-analysis. ERJ Open Res. 2021, 7, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ureña, J.F.; Ebersol, L.A.; Silakov, A.; Elias, R.J.; Lambert, J.D. Impact of Atomizer Age and Flavor on In Vitro Toxicity of Aerosols from a Third-Generation Electronic Cigarette against Human Oral Cells. Chem. Res. Toxicol. 2020, 33, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Kapan, A.; Stefanac, S.; Sandner, I.; Haider, S.; Grabovac, I.; Dorner, T.E. Use of Electronic Cigarettes in European Populations: A Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, M.; Krzystanek, M.; Zejda, J.E.; Majek, P.; Lubanski, J.; Lawson, J.A.; Brozek, G. E-Cigarettes are More Addictive than Traditional Cigarettes-A Study in Highly Educated Young People. Int. J. Environ. Res. Public Health 2019, 16, 2279. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, M.; Minarowski, Ł.; Mróz, R.M.; Guziejko, K.; Mojsak, D.; Poznański, M.; Zielonka, T.M.; Rachel, M.; Kornicki, K.; Pepłowska, P.; et al. E-cigarette use among young adults in Poland: Prevalence and characteristics of e-cigarette users. Adv. Med. Sci. 2020, 65, 437–441. [Google Scholar] [CrossRef]
- Zwar, N.A. Smoking cessation. Aust. J. Gen. Pract. 2020, 49, 474–481. [Google Scholar] [CrossRef]
- Marcham, C.L.; Springston, J.P. Electronic cigarettes in the indoor environment. Rev. Environ. Health 2019, 34, 105–124. [Google Scholar] [CrossRef] [Green Version]
- Bals, R.; Boyd, J.; Esposito, S.; Foronjy, R.; Hiemstra, P.S.; Jiménez-Ruiz, C.A.; Katsaounou, P.; Lindberg, A.; Metz, C.; Schober, W.; et al. Electronic cigarettes: A task force report from the European Respiratory Society. Eur. Respir J. 2019, 53, 1801151. [Google Scholar] [CrossRef] [Green Version]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef] [PubMed]
- Almeida-da-Silva, C.L.C.; Matshik Dakafay, H.; O’Brien, K.; Montierth, D.; Xiao, N.; Ojcius, D.M. Effects of electronic cigarette aerosol exposure on oral and systemic health. Biomed. J. 2021, 44, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ramôa, C.P.; Eissenberg, T.; Sahingur, S.E. Increasing popularity of waterpipe tobacco smoking and electronic cigarette use: Implications for oral healthcare. J. Periodontal. Res. 2017, 52, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Toy, J.; Dong, F.; Lee, C.; Zappa, D.; Le, T.; Archambeau, B.; Culhane, J.T.; Neeki, M.M. Alarming increase in electronic nicotine delivery systems-related burn injuries: A serious unregulated public health issue. Am. J. Emerg. Med. 2017, 35, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.K.; Kleinman, J.W.; Brooks, J.B.; Reynolds, M.A. Electronic cigarette explosion associated with extensive intraoral injuries. Dent. Traumatol. 2017, 33, 149–152. [Google Scholar] [CrossRef]
- Ebersole, J.; Samburova, V.; Son, Y.; Cappelli, D.; Demopoulos, C.; Capurro, A.; Pinto, A.; Chrzan, B.; Kingsley, K.; Howard, K.; et al. Harmful chemicals emitted from electronic cigarettes and potential deleterious effects in the oral cavity. Tob. Induc. Dis. 2020, 18, 41. [Google Scholar] [CrossRef]
- Zervas, E.; Matsouki, N.; Kyriakopoulos, G.; Poulopoulos, S.; Ioannides, T.; Katsaounou, P. Transfer of metals in the liquids of electronic cigarettes. Inhal. Toxicol. 2020, 32, 240–248. [Google Scholar] [CrossRef]
- Uchiyama, S.; Noguchi, M.; Sato, A.; Ishitsuka, M.; Inaba, Y.; Kunugita, N. Determination of Thermal Decomposition Products Generated from E-Cigarettes. Chem. Res. Toxicol. 2020, 33, 576–583. [Google Scholar] [CrossRef]
- Klein, M.D.; Sokol, N.A.; Stroud, L.R. Electronic Cigarettes: Common Questions and Answers. Am. Fam. Physician. 2019, 100, 227–235. [Google Scholar]
- Geiss, O.; Bianchi, I.; Barahona, F.; Barrero-Moreno, J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int. J. Hyg. Environ. Health 2015, 218, 169–180. [Google Scholar] [CrossRef]
- Shao, X.M.; Friedman, T.C. Pod-mod vs. conventional e-cigarettes: Nicotine chemistry, pH, and health effects. J. Appl. Physiol. 2020, 128, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, D.L.; Kass, A.P.; Chiel, L.E.; Boyer, E.W.; Casey, A.M.H. A review of toxic effects of electronic cigarettes/vaping in adolescents and young adults. Crit. Rev. Toxicol. 2020, 50, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Harvanko, A.M.; Havel, C.M.; Jacob, P.; Benowitz, N.L. Characterization of Nicotine Salts in 23 Electronic Cigarette Refill Liquids. Nicotine Tob. Res. 2020, 22, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Boykan, R.; Messina, C.R.; Eliscu, A.; Tolentino, J. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob. Control. 2019, 28, 676–677. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.; Heckman, B.W.; Wahlquist, A.E.; Cummings, K.M.; Carpenter, M.J. The Impact of E-liquid Propylene Glycol and Vegetable Glycerin Ratio on Ratings of Subjective Effects, Reinforcement Value, and Use in Current Smokers. Nicotine Tob. Res. 2020, 22, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Khlystov, A.; Samburova, V. Flavoring Compounds Dominate Toxic Aldehyde Production during e-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Samburova, V.; Bhattarai, C.; Strickland, M.; Darrow, L.; Angermann, J.; Son, Y.; Khlystov, A. Aldehydes in Exhaled Breath during e-Cigarette Vaping: Pilot Study Results. Toxics 2018, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Beauval, N.; Verriele, M.; Garat, A.; Fronval, I.; Dusautoir, R.; Antherieu, S.; Garcon, G.; Lo-Guidice, J.M.; Allorge, D.; Locoge, N. Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols. Int. J. Hyg. Environ. Health 2019, 222, 136–146. [Google Scholar] [CrossRef]
- Mara, A.; Langasco, I.; Deidda, S.; Caredda, M.; Meloni, P.; Deroma, M.; Pilo, M.I.; Spano, N.; Sanna, G. ICP-MS Determination of 23 Elements of Potential Health Concern in Liquids of e-Cigarettes. Method Development, Validation, and Application to 37 Real Samples. Molecules 2021, 26, 6680. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, N.; Gray, N.; Pappas, R.S.; Halstead, M.; Lewis, E.; Valentin-Blasini, L.; Watson, C.; Blount, B. Analysis of Toxic Metals in Aerosols from Devices Associated with Electronic Cigarette, or Vaping, Product Use Associated Lung Injury. Toxics 2021, 9, 240. [Google Scholar] [CrossRef]
- Landmesser, A.; Scherer, M.; Scherer, G.; Sarkar, M.; Edmiston, J.S.; Niessner, R.; Pluym, N. Assessment of the potential vaping-related exposure to carbonyls and epoxides using stable isotope-labeled precursors in the e-liquid. Arch. Toxicol. 2021, 95, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Beauval, N.; Antherieu, S.; Soyez, M.; Gengler, N.; Grova, N.; Howsam, M.; Hardy, E.M.; Fischer, M.; Appenzeller, B.M.R.; Goossens, J.F.; et al. Chemical Evaluation of Electronic Cigarettes: Multicomponent Analysis of Liquid Refills and their Corresponding Aerosols. J. Anal. Toxicol. 2017, 41, 670–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halstead, M.; Gray, N.; Gonzalez-Jimenez, N.; Fresquez, M.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of Toxic Metals in Electronic Cigarette Aerosols Using a Novel Trap Design. J. Anal. Toxicol. 2020, 44, 149–155. [Google Scholar] [CrossRef]
- Pappas, R.S.; Gray, N.; Halstead, M.; Valentin-Blasini, L.; Watson, C. Toxic Metal-Containing Particles in Aerosols from Pod-Type Electronic Cigarettes. J. Anal. Toxicol. 2021, 45, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.W.; Newmeyer, M.N.; Rule, A.M.; Prasse, C. Characterizing the Chemical Landscape in Commercial E-Cigarette Liquids and Aerosols by Liquid Chromatography-High-Resolution Mass Spectrometry. Chem. Res. Toxicol. 2021, 34, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Elias, R.J.; Silakov, A.; Foulds, J.; Muscat, J.; Richie, J.P., Jr. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 2018, 120, 72–79. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Foulds, J.; Muscat, J.; Elias, R.J.; Richie, J.P., Jr. Effects of Solvent and Temperature on Free Radical Formation in Electronic Cigarette Aerosols. Chem. Res. Toxicol. 2018, 31, 4–12. [Google Scholar] [CrossRef]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef] [Green Version]
- Shein, M.; Jeschke, G. Comparison of Free Radical Levels in the Aerosol from Conventional Cigarettes, Electronic Cigarettes, and Heat-Not-Burn Tobacco Products. Chem. Res. Toxicol. 2019, 32, 1289–1298. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Goel, R.; Trushin, N.; Muscat, J.; Richie, J.P., Jr. Free Radical Production and Characterization of Heat-Not-Burn Cigarettes in Comparison to Conventional and Electronic Cigarettes. Chem. Res. Toxicol. 2020, 33, 1882–1887. [Google Scholar] [CrossRef]
- Yogeswaran, S.; Muthumalage, T.; Rahman, I. Comparative Reactive Oxygen Species (ROS) Content among Various Flavored Disposable Vape Bars, including Cool (Iced) Flavored Bars. Toxics 2021, 9, 235. [Google Scholar] [CrossRef]
- Barhdadi, S.; Mertens, B.; van Bossuyt, M.; van de Maele, J.; Anthonissen, R.; Canfyn, M.; Courselle, P.; Rogiers, V.; Deconinck, E.; Vanhaecke, T. Identification of flavouring substances of genotoxic concern present in e-cigarette refills. Food Chem. Toxicol. 2021, 147, 111864. [Google Scholar] [CrossRef] [PubMed]
- Margham, J.; McAdam, K.; Cunningham, A.; Porter, A.; Fiebelkorn, S.; Mariner, D.; Digard, H.; Proctor, C. The Chemical Complexity of e-Cigarette Aerosols Compared With the Smoke From a Tobacco Burning Cigarette. Front. Chem. 2021, 9, 743060. [Google Scholar] [CrossRef] [PubMed]
- Tantisuwat, A.; Thaveeratitham, P. Effects of smoking on chest expansion, lung function, and respiratory muscle strength of youths. J. Phys. Ther. Sci. 2014, 26, 167–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140. [Google Scholar] [CrossRef] [PubMed]
- Kopa, P.N.; Pawliczak, R. Effect of smoking on gene expression profile-overall mechanism, impact on respiratory system function, and reference to electronic cigarettes. Toxicol. Mech. Methods 2018, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Rowell, T.R.; Reeber, S.L.; Lee, S.L.; Harris, R.A.; Nethery, R.C.; Herring, A.H.; Glish, G.L.; Tarran, R. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L52–Ll66. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Chiu, W.A.; Cogliano, V.; Jinot, J.; Kriebel, D.; Lunn, R.M.; Beland, F.A.; Bero, L.; Browne, P.; Fritschi, L.; et al. The IARC Monographs: Updated Procedures for Modern and Transparent Evidence Synthesis in Cancer Hazard Identification. J. Natl. Cancer Inst. 2020, 112, 30–37. [Google Scholar] [CrossRef]
- Stratton, K.; Kwan, L.Y.; Eaton, D.L. Public Health Consequences of E-Cigarettes; The National Academies Press: Washington, DC, USA, 2018; pp. 455–460. [Google Scholar]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef]
- Ralho, A.; Coelho, A.; Ribeiro, M.; Paula, A.; Amaro, I.; Sousa, J.; Marto, C.; Ferreira, M.; Carrilho, E. Effects of Electronic Cigarettes on Oral Cavity: A Systematic Review. J. Evid. Based Dent. Pract. 2019, 19, 101318. [Google Scholar] [CrossRef]
- Etter, J.F. Electronic cigarettes: A survey of users. BMC Public Health 2010, 10, 231. [Google Scholar] [CrossRef]
- Etter, J.F.; Bullen, C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction 2011, 106, 2017–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, D.; Gajendra, S.; Lawyer, G.; Jadeja, N.; Pishey, D.; Pathagunti, S.; Lyons, J.; Veazie, P.; Watson, G.; McIntosh, S.; et al. Inflammatory biomarkers and growth factors in saliva and gingival crevicular fluid of e-cigarette users, cigarette smokers, and dual smokers: A pilot study. J. Periodontol. 2020, 91, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Cichońska, D.; Król, O.; Słomińska, E.M.; Kochańska, B.; Świetlik, D.; Ochocińska, J.; Kusiak, A. Influence of Electronic Cigarettes on Antioxidant Capacity and Nucleotide Metabolites in Saliva. Toxics 2021, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000, 84, 45–68. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease-Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef] [Green Version]
- Gare, J.; Kanoute, A.; Meda, N.; Viennot, S.; Bourgeois, D.; Carrouel, F. Periodontal Conditions and Pathogens Associated with Pre-Eclampsia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 7194. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. The Oral Microbiota in Health and Disease: An Overview of Molecular Findings. Methods Mol. Biol. 2017, 1537, 127–138. [Google Scholar] [PubMed]
- Kubica, P.; Wasik, A.; Kot-Wasik, A.; Namieśnik, J. An evaluation of sucrose as a possible contaminant in e-liquids for electronic cigarettes by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 3013–3018. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Clark, P.; Brinkman, M.C.; Saxena, D. Novel Nicotine Delivery Systems. Adv. Dent. Res. 2019, 30, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Paul, B.; Li, Q.; Yang, J.; Vasconcelos, R.; Makwana, S.; González, J.M.; Shah, S.; Xie, C.; Janal, M.N.; et al. Electronic Cigarette Aerosol Modulates the Oral Microbiome and Increases Risk of Infection. iScience 2020, 23, 100884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopyk, J.; Bojanowski, C.M.; Shin, J.; Moshensky, A.; Fuentes, A.L.; Bonde, S.S.; Chuki, D.; Pride, D.T.; Crotty, A.L.E. Compositional Differences in the Oral Microbiome of E-cigarette Users. Front Microbiol. 2021, 12, 599664. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Cherian, D.A.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a Marker of Oxidative Stress in Periodontitis Patients. J. Pharm. Bioallied. Sci. 2019, 11, S297–S300. [Google Scholar] [CrossRef]
- Upadhyay, M.; Verma, P.; Sabharwal, R.; Subudhi, S.K.; Jatol-Tekade, S.; Naphade, V.; Choudhury, B.K.; Sahoo, P.D. Micronuclei in Exfoliated Cells: A Biomarker of Genotoxicity in Tobacco Users. Niger J. Surg. 2019, 25, 52–59. [Google Scholar]
- Menicagli, R.; Marotta, O.; Serra, R. Free radical production in the smoking of e-cigarettes and their possible effects in human health. Int. J. Prev. Med. 2020, 11, 53. [Google Scholar]
- Tommasi, S.; Caliri, A.W.; Caceres, A.; Moreno, D.E.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. Deregulation of Biologically Significant Genes and Associated Molecular Pathways in the Oral Epithelium of Electronic Cigarette Users. Int. J. Mol. Sci. 2019, 20, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardellini, E.; Amadori, F.; Conti, G.; Majorana, A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol. Scand. 2018, 76, 226–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuther, W.J.; Hale, B.; Matharu, J.; Blythe, J.N.; Brennan, P.A. Do you mind if I vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue—A pilot study. Br. J. Oral Maxillofac. Surg. 2016, 54, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.M.; Kabir, Z. Burn injuries caused by e-cigarette explosions: A systematic review of published cases. Tob. Prev. Cessat. 2018, 4, 32. [Google Scholar] [CrossRef]
- Yang, I.; Sandeep, S.; Rodriguez, J. The oral health impact of electronic cigarette use: A systematic review. Crit Rev. Toxicol. 2020, 50, 1–31. [Google Scholar] [CrossRef]
- Harrison, R.; Hicklin, D., Jr. Electronic cigarette explosions involving the oral cavity. J. Am. Dent. Assoc. 2016, 147, 891–896. [Google Scholar] [CrossRef]
- Rogér, J.M.; Abayon, M.; Elad, S.; Kolokythas, A. Oral Trauma and Tooth Avulsion Following Explosion of E-Cigarette. J. Oral. Maxillofac. Surg. 2016, 74, 1181–1185. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.S.; Jessri, M.; Farah, C.S. Electronic nicotine delivery systems: Oral health implications and oral cancer risk. J. Oral Pathol. Med. 2021, 50, 316–322. [Google Scholar] [CrossRef]
- Bestman, E.G.; Brooks, J.K.; Mostoufi, B.; Bashirelahi, N. What every dentist needs to know about electronic cigarettes. Gen. Dent. 2021, 69, 31–35. [Google Scholar]
- La Valle, A.; O’Connor, R.; Brooks, A.; Freij, R. Maxillofacial injury related to an exploding e-cigarette. BMJ Case Rep. 2021, 14, e239677. [Google Scholar] [CrossRef]
| CAS Number | Component | Group |
|---|---|---|
| 75–07-0 | Acetaldehyd | 2B |
| 107–02-8 | Acreolin | 2A |
| 50–00-0 | Formaldehyde | 1 |
| 7439–92-1 | Lead | 2B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumilas, P.; Wilk, A.; Szumilas, K.; Karakiewicz, B. The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review. Toxics 2022, 10, 74. https://doi.org/10.3390/toxics10020074
Szumilas P, Wilk A, Szumilas K, Karakiewicz B. The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review. Toxics. 2022; 10(2):74. https://doi.org/10.3390/toxics10020074
Chicago/Turabian StyleSzumilas, Paweł, Aleksandra Wilk, Kamila Szumilas, and Beata Karakiewicz. 2022. "The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review" Toxics 10, no. 2: 74. https://doi.org/10.3390/toxics10020074
APA StyleSzumilas, P., Wilk, A., Szumilas, K., & Karakiewicz, B. (2022). The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review. Toxics, 10(2), 74. https://doi.org/10.3390/toxics10020074