Toxicokinetics of Chromium in Enchytraeus crypticus (Oligochaeta)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Test Soil
2.3. Test Substance and Spiking Procedures
2.4. Experimental Procedure
2.5. Chemical Analysis
2.6. Data Analysis
3. Results
3.1. Soil Analysis
3.2. Organism Weight
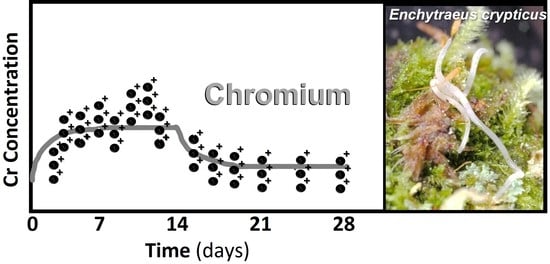
3.3. Toxicokinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Barceloux, D. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Bielicka, A.; Bojanowska, I.; Wiśniewski, A. Two faces of chromium—Pollutant and bioelement. Pol. J. Environ. Stud. 2005, 14, 5–10. [Google Scholar]
- Fendorf, S.E. Surface reactions of chromium in soils and waters. Geoderma 1995, 67, 55–71. [Google Scholar] [CrossRef]
- Shanker, A.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Baruthio, F. Toxic effects of chromium and its compounds. Biol. Trace Elem. Res. 1992, 32, 145–153. [Google Scholar] [CrossRef]
- Sivakumar, S.; Subbhuraam, C.V. Toxicity of chromium(III) and chromium(VI) to the earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2005, 62, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Chae, Y. Life Cycle Parameters of the Earthworm Eisenia fetida Exposed to Cr(III) and Cr(VI) Amended Organic Substrates. In Dynamic Soil, Dynamic Plant; Global Science Books: Carrollton, GA, USA, 2009; pp. 147–153. ISBN 978-4-903313-39-9. [Google Scholar]
- Bigorgne, E.; Cossu-Leguille, C.; Bonnard, M.; Nahmani, J. Genotoxic effects of nickel, trivalent and hexavalent chromium on the Eisenia fetida earthworm. Chemosphere 2010, 80, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, B.; Utpal, S.; Mukhopadhyay, S. Mobility and bioavailability of chromium in the environment: Physico-chemical and microbial oxidation of Cr (III) to Cr (VI). J. Appl. Sci. Environ. Manag. 2010, 14. [Google Scholar] [CrossRef]
- Cao, X.; Guo, J.; Mao, J.; Lan, Y. Adsorption and mobility of Cr(III)–organic acid complexes in soils. J. Hazard. Mater. 2011, 192, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Avudainayagam, S.; Megharaj, M.; Owens, G.; Kookana, R.S.; Chittleborough, D.; Naidu, R. Chemistry of chromium in soils with emphasis on tannery waste sites. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2003; Volume 178, pp. 53–91. ISBN 0387217282. [Google Scholar]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A. Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Z.; Zhao, L.; Ma, J.; Li, X.; He, F.; Hou, H. Toxicity of exogenous hexavalent chromium to soil-dwelling springtail Folsomia candida in relation to soil properties and aging time. Chemosphere 2019, 224, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Aebeed, A.S.; Mohamed, A.I. Effect of some heavy metals on the growth, cocoon production and juvenile number of the earthworm Eisenia fetida. Libyan J. Sci. Technol. 2018, 7, 91–95. [Google Scholar]
- Lock, K.; Janssen, C. Ecotoxicity of chromium (III) to Eisenia fetida, Enchytraeus albidus, and Folsomia candida. Ecotoxicol. Environ. Saf. 2002, 51, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Lock, K.; Janssen, C.R.R. Mixture toxicity of zinc, cadmium, copper, and lead to the potworm Enchytraeus albidus. Ecotoxicol. Environ. Saf. 2002, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Basha, P.M.; Latha, V. Evaluation of sublethal toxicity of zinc and chromium in Eudrilus eugeniae using biochemical and reproductive parameters. Ecotoxicology 2016, 25, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.J.; Satyanarayan, S. Effect of selected heavy metals on the histopathology of different tissues of earthworm Eudrillus eugeniae. Environ. Monit. Assess. 2011, 180, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Didden, W.; Römbke, J. Enchytraeids as indicator organisms for chemical stress in terrestrial ecosystems. Ecotoxicol. Environ. Saf. 2001, 50, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Jänsch, S.; Amorim, M.J.; Römbke, J. Identification of the ecological requirements of important terrestrial ecotoxicological test species. Environ. Rev. 2005, 13, 51–83. [Google Scholar] [CrossRef]
- Jager, T.; Albert, C.; Preuss, T.G.; Ashauer, R. General Unified Threshold Model of survival—A toxicokinetic-toxicodynamic framework for ecotoxicology. Environ. Sci. Technol. 2011, 45, 2529–2540. [Google Scholar] [CrossRef]
- Newman, M.C. Fundamentals of Ecotoxicology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9781351133999. [Google Scholar]
- Jager, T.; Ashauer, R. How to evaluate the quality of toxicokinetic-toxicodynamic models in the context of environmental risk assessment. Integr. Environ. Assess. Manag. 2018, 14, 604–614. [Google Scholar] [CrossRef]
- Kilpi-Koski, J.; Penttinen, O.-P.; Väisänen, A.O.; van Gestel, C.A.M. An uptake and elimination kinetics approach to assess the bioavailability of chromium, copper, and arsenic to earthworms (Eisenia andrei) in contaminated field soils. Environ. Sci. Pollut. Res. 2019, 26, 15095–15104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gestel, C.A.M.; Dirven-van Breemen, E.M.; Baerselman, R. Accumulation and elimination of cadmium, chromium and zinc and effects on growth and reproduction in Eisenia andrei (Oligochaeta, Annelida). Sci. Total Environ. 1993, 134, 585–597. [Google Scholar] [CrossRef]
- He, E.; van Gestel, C.A.M. Toxicokinetics and toxicodynamics of nickel in Enchytraeus crypticus. Environ. Toxicol. Chem. 2013, 32, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.F.; van Gestel, C.A.M.; Amorim, M.J.B. Toxicokinetics of copper and cadmium in the soil model Enchytraeus crypticus (Oligochaeta). Chemosphere 2021, 270, 129433. [Google Scholar] [CrossRef]
- Zhang, L.; Van Gestel, C.A.M. Toxicokinetics and toxicodynamics of lead in the soil invertebrate Enchytraeus crypticus. Environ. Pollut. 2017, 225, 534–541. [Google Scholar] [CrossRef]
- Santos, F.C.F.; Tourinho, P.S.; Scott-Fordsmand, J.J.; van Gestel, C.A.M.; Amorim, M.J.B. Toxicokinetics of Ag (nano)materials in the soil model Enchytraeus crypticus (Oligochaeta)—Impact of aging and concentration. Environ. Sci. Nano 2021, 8, 2629–2640. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Hopkin, S.P. Comparisons of metal accumulation and excretion kinetics in earthworms (Eisenia fetida) exposed to contaminated field and laboratory soils. Appl. Soil Ecol. 1999, 11, 227–243. [Google Scholar] [CrossRef]
- Santos, F.C.F.; Van Gestel, C.A.M.; Amorim, M.J.B. Impact of chromium on the soil invertebrate model Enchytraeus crypticus (Oligochaeta) in standard reproduction and full life cycle tests. Chemosphere 2021, 291, 132751. [Google Scholar] [CrossRef] [PubMed]
- Westheide, W.; Graefe, U. Two new terrestrial Enchytraeus species (Oligochaeta, Annelida). J. Nat. Hist. 1992, 26, 479–488. [Google Scholar] [CrossRef]
- OECD. Test No. 317: Bioaccumulation in Terrestrial Oligochaetes; OECD Guidelines for the Testing of Chemicals, Section 3; Organization for Economic Co-Operation and Development: Paris, France, 2010; ISBN 9789264090934. [Google Scholar]
- ISO 6341; Determination of the Inhibition of the Mobility of DAPHNIA Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Standardization Organization: Geneva, Swizerland, 2012.
- Ardestani, M.M.; van Straalen, N.M.; van Gestel, C.A.M. Uptake and elimination kinetics of metals in soil invertebrates: A review. Environ. Pollut. 2014, 193, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Peijnenburg, W.J.G.M.; Baerselman, R.; de Groot, A.C.; Jager, T.; Posthuma, L.; Van Veen, R.P.M. Relating environmental availability to bioavailability: Soil-type-dependent metal accumulation in the Oligochaete Eisenia andrei. Ecotoxicol. Environ. Saf. 1999, 44, 294–310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Verweij, R.A.; Van Gestel, C.A.M. Effect of soil properties on Pb bioavailability and toxicity to the soil invertebrate Enchytraeus crypticus. Chemosphere 2019, 217, 9–17. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Lister, L.; Kille, P.; Pereira, M.G.; Wright, J.; Svendsen, C. Toxicokinetic studies reveal variability in earthworm pollutant handling. Pedobiologia 2011, 54, S217–S222. [Google Scholar] [CrossRef]
- Zhang, L.; Belloc da Silva Muccillo, V.; Van Gestel, C.A.M. A combined toxicokinetics and toxicodynamics approach to investigate delayed lead toxicity in the soil invertebrate Enchytraeus crypticus. Ecotoxicol. Environ. Saf. 2019, 169, 33–39. [Google Scholar] [CrossRef]
- Crommentuijn, T.; Doodeman, C.J.A.M.; Doornekamp, A.; Van Der Pol, J.J.C.; Van Gestel, C.A.M.; Bedaux, J.J.M. Lethal body concentrations and accumulation patterns determine time-dependent toxicity of cadmium in soil arthropods. Environ. Toxicol. Chem. 1994, 13, 1781–1789. [Google Scholar] [CrossRef]
- Shahmansouri, M.R.; Pourmoghadas, H.; Parvaresh, A.; Alidadi, A. Heavy metals bioaccumulation by Iranian and Australian earthworms (Eisenia fetida) in the sewage sludge vermicomposting. Indian J. Env. Health Sci Eng. 2005, 2, 28–32. [Google Scholar]
- Nahmani, J.; Hodson, M.E.; Devin, S.; Vijver, M.G. Uptake kinetics of metals by the earthworm Eisenia fetida exposed to field-contaminated soils. Environ. Pollut. 2009, 157, 2622–2628. [Google Scholar] [CrossRef]
- Giska, I.; van Gestel, C.A.M.; Skip, B.; Laskowski, R. Toxicokinetics of metals in the earthworm Lumbricus rubellus exposed to natural polluted soils—Relevance of laboratory tests to the field situation. Environ. Pollut. 2014, 190, 123–132. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Rainbow, P. Regulation and accumulation of copper, zinc and cadmium by the shrimp Palaemon elegans. Mar. Ecol. Prog. Ser. 1982, 8, 95–101. [Google Scholar] [CrossRef]
- Chan, H.M.; Rainbow, P.S. On the excretion of zinc by the shore crab Carcinus maenas (L.). Ophelia 1993, 38, 31–45. [Google Scholar] [CrossRef]
- Lanno, R.; Wells, J.; Conder, J.; Bradham, K.; Basta, N. The bioavailability of chemicals in soil for earthworms. Ecotoxicol. Environ. Saf. 2004, 57, 39–47. [Google Scholar] [CrossRef] [PubMed]


| Nominal (mg Cr/kg Soil DW) | pH (CaCl2) | Measured Total (mg Cr/kg Soil DW) (Recovery) | CaCl2 Extractable (mg Cr/kg Soil DW) | Survival (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time (days) | 0 | 28 | 0 | 1 | 14 | 0 | 1 | 14 | 28 |
| 0 | 5.5 | 5.2 | 14 ± 1.2 | - | 13.8 ± 0.4 | 0.14 ± 0.0 | - | 0.12 ± 0.0 | 88 ± 5.8 |
| 180 | 5.5 | 5.2 | 147 ± 2.8 (81%) | 139 ± 6 (77%) | 148 ± 3.4 (82%) | 0.25 ± 0.2 | 0.15 ± 0.0 | 0.16 ± 0.0 | 94 ± 5.8 |
| Parameter | Total | CaCl2 |
|---|---|---|
| Cexp (mg/kg dry soil) | 145 | 0.15 * |
| C0 (mg/kg dry body wt.) (AV ± SE) | 4.6 ± 0.4 | |
| Ku (kg soil/kg animal/day) | 0.012 [−0.06,0.3] | 12 [−16.7,40.7] |
| Ke (day−1) | 0.57 [−0.5,1.6] | 0.57 [−0.5,1.6] |
| DT50 (days) | 1.2 | |
| Kgrowth–uptake phase (day−1) | 0.061 | |
| Kgrowth–elimination phase (day−1) | −0.019 | |
| BAF | 0.022 | 21.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.C.F.; Verweij, R.A.; van Gestel, C.A.M.; Amorim, M.J.B. Toxicokinetics of Chromium in Enchytraeus crypticus (Oligochaeta). Toxics 2022, 10, 82. https://doi.org/10.3390/toxics10020082
Santos FCF, Verweij RA, van Gestel CAM, Amorim MJB. Toxicokinetics of Chromium in Enchytraeus crypticus (Oligochaeta). Toxics. 2022; 10(2):82. https://doi.org/10.3390/toxics10020082
Chicago/Turabian StyleSantos, Fátima C. F., Rudo A. Verweij, Cornelis A. M. van Gestel, and Mónica J. B. Amorim. 2022. "Toxicokinetics of Chromium in Enchytraeus crypticus (Oligochaeta)" Toxics 10, no. 2: 82. https://doi.org/10.3390/toxics10020082
APA StyleSantos, F. C. F., Verweij, R. A., van Gestel, C. A. M., & Amorim, M. J. B. (2022). Toxicokinetics of Chromium in Enchytraeus crypticus (Oligochaeta). Toxics, 10(2), 82. https://doi.org/10.3390/toxics10020082