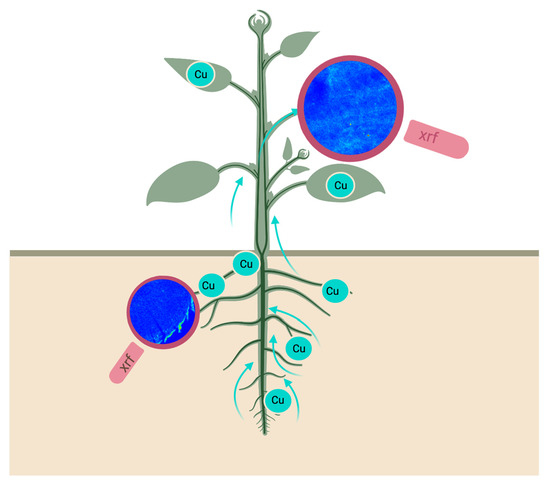
Enlightening the Pathway of Phytoremediation: Ecophysiology and X-ray Fluorescence Visualization of Two Chilean Hardwoods Exposed to Excess Copper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Physiological Measurements
2.3. Element Visualization
2.4. Statistics
3. Results
3.1. Soil Mix
3.2. Growth
3.3. Photosynthesis
3.4. Elemental Analysis
3.5. X-ray Fluorescence Microimaging
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sáez, A.; Choe, H.; Boynton, R.M.; Elsen, P.R.; Thorne, J.H. Climate exposure shows high risk and few climate refugia for Chilean native vegetation. Sci. Total Environ. 2021, 785, 147399. [Google Scholar] [CrossRef]
- PUCV. Available online: https://www.pucv.cl/uuaa/vriea/noticias/nuestros-investigadores/en-chile-diariamente-se-desecha-cobre-avaluado-en-una-cifra-cercana-a (accessed on 24 February 2022).
- DatosGob. Available online: https://datos.gob.cl/dataset/ley-promedio-de-mineral-de-cobre-en-las-operaciones-mineras-en-chile/resource/557313a1-fb08-418d-b636-14a3029670e5 (accessed on 11 September 2021).
- Haas, J.; Moreno-Leiva, S.; Junne, T.; Chen, P.J.; Pamparana, G.; Nowak, W.; Kracht, W.; Ortiz, J.M. Copper mining: 100% solar electricity by 2030? Appl. Energy 2020, 262, 114506. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Ortiz-Calderón, C.; Alcaide, Ó.; Kao, J.L. Copper distribution in leaves and roots of plants on a copper mine-tailing storage facility in northern Chile. Rev. Chil. Hist. Nat. 2008, 81, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Cárcamo, V.; Bustamante, E.; Trangolao, E.; de la Fuente, L.M.; Mench, M.; Neaman, A.; Ginocchio, R. Simultaneous immobilization of metals and arsenic in acidic polluted soils near a copper smelter in central Chile. Environ. Sci. Pollut. Res. 2012, 19, 1131–1143. [Google Scholar] [CrossRef]
- Meza-Ramírez, V.; Espinoza-Ortiz, X.; Ramírez-Verdugo, P.; Hernández-Lazcano, P.; Hermosilla, P.R. Pb-contaminated soil from Quintero-Ventanas, Chile: Remediation using Sarcocornia neei. Sci. World J. 2021, 2021, 2974786. [Google Scholar] [CrossRef] [PubMed]
- Orchard, C.; León-Lobos, P.; Ginocchio, R. Phytostabilization of massive mine wastes with native phytogenetic resources: Potential for sustainable use and conservation of the native flora in north-central Chile. Cien. Inv. Agr. 2009, 36, 329–352. [Google Scholar] [CrossRef]
- Milla-Moreno, E.; Guy, R.D. Growth response, uptake and mobilization of metals in native plant species on tailings at a Chilean copper mine. Int. J. Phytoremediation 2020, 23, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Ginocchio, R.; Toro, I.; Schnepf, D.; Macnair, M.R. Copper tolerance testing in populations of Mimulus luteus var. variegatus exposed and non-exposed to copper mine pollution. Geochem. Explor. Environ. Anal. 2002, 2, 151–156. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Vijayan, P.; Willick, I.R.; Lahlali, R.; Karunakaran, C.; Tanino, K.K. Synchrotron radiation sheds fresh light on plant research: The use of powerful techniques to probe structure and composition of plants. Plant Cell Physiol. 2015, 56, 1252–1263. [Google Scholar] [CrossRef]
- Cervantes-Garcia, D.; Rubalcaba-Sil, J.L.; Gonzalez-Mendoza, D.; Avilés-Marín, M. Assessment of copper bioaccumulation in Euglena gracilis by X-ray fluorescence technique. Lat. Am. J. Aquat. Res. 2014, 42, 662–665. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Zhang, J.; Wang, S.; White, J.C.; Chen, G.; Xing, B. Accumulation and spatial distribution of copper and nutrients in willow as affected by soil flooding: A synchrotron-based X-ray fluorescence study. Environ. Pollut. 2019, 246, 980–989. [Google Scholar] [CrossRef]
- Willick, I.R.; Lahlali, R.; Vijayan, P.; Muir, D.; Karunakaran, C.; Tanino, K.K. Wheat flag leaf epicuticular wax morphology and composition in response to moderate drought stress are revealed by SEM, FTIR-ATR and synchrotron X-ray spectroscopy. Physiol. Plant. 2018, 162, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Susana, B.R. (Ed.) INFOR: Monografía de espino Acacia caven (Mol.) Mol. In Programa de Investigación de Productos Forestales No Madereros; Ministerio de Agricultura. Gobierno de Chile: Santiago, Chile, 2012; pp. 24–28. [Google Scholar]
- INFOR: Quillay Quillaja saponaria. Available online: http://transparencia.minagri.gob.cl/descargas/2011/medios/INFOR/ficha_quillay_Metodo_de_propagacion.pdf (accessed on 2 September 2018).
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Moghanm, F.S.; Youssef, M.S.G.; El-Mohsnawy, E.; Haroun, S.A. Bioaccumulation and translocation of nine heavy metals by Eichhornia crassipes in Nile Delta, Egypt: Perspectives for phytoremediation. Int. J. Phytoremediation 2019, 21, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Canadian Light Source. Available online: https://bioxas-imaging.lightsource.ca/ (accessed on 2 December 2021).
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- R Core Team. Available online: https://www.r-project.org/ (accessed on 30 October 2018).
- Kumar, V.; Pandita, S.; Singh-Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Bouazizi, H.; Jouili, H.; Geitmann, A.; El Ferjani, E. Copper toxicity in expanding leaves of Phaseolus vulgaris L.: Antioxidant enzyme response and nutrient element uptake. Ecotoxicol. Environ. Saf. 2010, 73, 1304–1308. [Google Scholar] [CrossRef]
- Ginocchio, R.; Sánchez, P.; de la Fuente, L.M.; Camus, I.; Bustamante, E.; Silva, Y.; Urrestarazu, P.; Torres, J.C.; Rodríguez, P.H. Agricultural soils spiked with copper mine wastes and copper concentrate: Implications for copper bioavailability and bioaccumulation. Environ. Toxicol. Chem. 2006, 25, 712–718. [Google Scholar] [CrossRef]
- Aguilar, R.; Hormazábal, C.; Gaete, H.; Neaman, A. Spatial distribution of copper, organic matter and pH in agricultural soils affected by mining activities. J. Soil Sci. Plant Nutr. 2011, 125–145. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Available online: https://ccme.ca/en/results/71/ch/1,2,3,4,5,6 (accessed on 22 February 2017).
- De la Iglesia, R.; Castro, D.; Ginocchio, R.; Van der Lelie, D.; González, B. Factors influencing the composition of bacterial communities found at abandoned copper-tailings dumps. J. Appl. Microbiol. 2006, 100, 537–544. [Google Scholar] [CrossRef]
- Alloway, B. Copper. In Heavy Metals in Soils:Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B., Trevors, J.T., Eds.; Springer: London, UK, 1995; pp. 367–394. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Copper. In Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 32, 98, 128, 137–212. [Google Scholar]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.; Hirano, Y.; Brunner, I. Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. Tree Physiol. 2007, 27, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lim, H.; Lee, I. Enhanced heavy metal phytoextraction by Echinochloa crus-galli using root exudates. J. Biosci. Bioeng. 2010, 109, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Alvear, M.; Borie, F.; Aguilera, P.; Ginocchio, R.; Cornejo, P. Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Maiti, S.K. Metal accumulation in A. baccifera growing naturally on abandoned copper tailings pond. Environ. Monit. Assess. 2007, 127, 119–125. [Google Scholar] [CrossRef]
- González, I.; Muena, V.; Cisternas, M.; Neaman, A. Acumulación de cobre en una comunidad vegetal afectada por contaminación minera en el valle de Puchuncaví, Chile central. Rev. Chil. Hist. Nat. 2008, 81, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Ginocchio, R.; Baker, A. Metallophytes in Latin America: A remarkable biological and genetic resource scarcely known and studied in the region. Rev. Chil. Hist. Nat. 2004, 77, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Lam, E.J.; Keith, B.F.; Montofré, Í.L.; Gálvez, M.E. Copper uptake by Adesmia atacamensis in a mine tailing in an arid environment. Air Soil Water Res. 2018, 11, 1178622118812462. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, R.; Flores, J.P.; Tapia, J.; Valdés-Pineda, R.; González, D.; Morales, C.; Sangüesa, C.; Balocchi, F.; León, L. Forest species in the recovery of soils contaminated with copper due to mining activities. Rev. Chapingo. 2016, 22, 29–43. [Google Scholar] [CrossRef]
- Mendel, R.R.; Hänsch, R. Molybdoenzymes and molybdenum cofactor in plants. J. Exp. Bot. 2002, 53, 1689–1698. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.J.M.; Ernst, W.H.O.; Van der Ent, A.; Malaisse, F.; Ginocchio, R. Metallophytes: The unique biological resource, its ecology and conservational status in Europe, Central Africa and Latin America. In Ecology of Industrial Pollution, 1st ed.; Lesley, K.B.H., Batty, C., Eds.; Cambridge University Press: Cambridge, UK, 2010; Volume 2, pp. 7–40. [Google Scholar]
- Weiss, W.P. Mineral tolerances of animals. In Proceedings of the Tri-State Dairy Nutrition Conference, Fort Wayne, IN, USA, 22–23 April 2008. [Google Scholar]
- Van der Ent, A.; Przybyłowicz, W.J.; de Jonge, M.D.; Harris, H.H.; Ryan, C.G.; Tylko, G.; Paterson, D.J.; Barnabas, A.D.; Kopittke, P.M.; Mesjasz-Przybyłowicz, J. X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants. New Phytol. 2018, 218, 432–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Shtangeeva, I. Bromine accumulation in some crops and grasses as determined by neutron activation analysis. Commun. Soil Sci. Plant Anal. 2017, 48, 2338–2346. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Garland, T.R.; Wildung, R.E. Nickel in plants. Uptake kinetics using intact Soybean seedlings. Plant Physiol. 1978, 62, 563–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Printz, B.; Lutts, S.; Hausman, J.F.; Sergeant, K. Copper trafficking in plants and its implication on cell wall dynamics. Front. Plant Sci. 2016, 7, 601. [Google Scholar] [CrossRef] [Green Version]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2010, 3, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.G.; Liu, W.; Yu, W.; Zhang, Y.; Ding, S.; Li, H.; Mrak, T.; Kraigher, H.; Luo, Z.B. Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus × canescens. J. Hazard. Mater. 2019, 362, 275–285. [Google Scholar] [CrossRef]
- Baccio, D.D.; Tognetti, R.; Minnocci, A.; Sebastiani, L. Responses of the Populus × euramericana clone I-214 to excess zinc: Carbon assimilation, structural modifications, metal distribution and cellular localization. Environ. Exp. Bot. 2009, 67, 153–163. [Google Scholar] [CrossRef]
- Hirano, Y.; Frey, B.; Brunner, I. Contrasting reactions of roots of two coniferous tree species to aluminum stress. Environ. Exp. Bot. 2012, 77, 12–18. [Google Scholar] [CrossRef]
- Cui, J.L.; Zhao, Y.P.; Chan, T.S.; Zhang, L.L.; Tsang, D.C.W.; Li, X.D. Spatial distribution and molecular speciation of copper in indigenous plants from contaminated mine sites: Implication for phytostabilization. J. Hazard. Mater. 2020, 381, 121208. [Google Scholar] [CrossRef] [PubMed]
- Van Hoof, N.A.; Hassinen, V.H.; Hakvoort, H.W.; Ballintijn, K.F.; Schat, H.; Verkleij, J.A.; Ernst, W.H.; Karenlampi, S.O.; Tervahauta, A.I. Enhanced copper tolerance in Silene vulgaris (Moench) Garcke populations from copper mines is associated with increased transcript levels of a 2b-type metallothionein gene. Plant Physiol. 2001, 126, 1519–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, W. Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015–2016; pp. 4–55. [Google Scholar]
- Lersten, N.R.; Horner, H.T. Macropattern of styloid and druse crystals in Quillaja (Quillajaceae) bark and leaves. Int. J. Plant Sci. 2005, 166, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Raman, V.; Horner, H.T.; Khan, I.A. New and unusual forms of calcium oxalate raphide crystals in the plant kingdom. J. Plant Res. 2014, 127, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Léon, V.; Rabier, J.; Notonier, R.; Barthelémy, R.; Moreau, X.; Bouraïma-Madjèbi, S.; Viano, J.; Pineau, R. Effects of three nickel salts on germinating seeds of Grevillea exul var. rubiginosa, an endemic serpentine proteaceae. Ann. Bot. 2005, 95, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Tissue | Species | Treatment | Ca (%) | Cu (mg/kg) | Fe (mg/kg) | K (%) | Mn (mg/kg) | Mo (mg/kg) | N (%) | Na (mg/kg) | P (%) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | |||
| [SD] | [SD] | [SD] | [SD] | [SD] | [SD] | [SD] | [SD] | [SD] | [SD] | |||
| Leaves | Espino | Control | 19,000 | 3.3 | 49 | 20,000 | 44 | 1.6 | 3.7 | 25 | 2900 | 15 |
| -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||
| 50 µM Cu | 14,000 | 8.7 | 53 | 11,000 | 34 | 2.9 | 3.5 | 29 | 1400 | 18 | ||
| -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||
| 100 µM Cu | 14,000 | 13 | 51 | 12,000 | 55 | 1.0 | 3.2 | 20 | 1500 | 16 | ||
| -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||
| Quillay | Control | 10,200 | 2.2 | 25.8 | 46,600 | 30 | 0.54 | 1.68 | 570 | 1860 | 15.8 | |
| [2470] | [1.2] | [1.8] | [9840] | [13] | [0.2] | [0.1] | [697] | [152] | [2.2] | |||
| 50 µM Cu | 12,800 | 10.1 | 25 | 43,200 | 48.6 | 1.2 | 1.6 | 320 | 2040 | 17.6 | ||
| [1480] | [3.7] | [6.3] | [2590] | [20.5] | [0.9] | [0.1] | [143] | [288] | [2.0] | |||
| 100 µM Cu | 9740 | 9.4 | 20.8 | 36,600 | 52 | 0.9 | 1.6 | 119 | 1940 | 15 | ||
| [3680] | [4.8] | [4.9] | [6190] | [30.9] | [0.5] | [0.2] | [40.4] | [611] | [2.5] | |||
| Roots | Espino | Control | 49,000 | 13 | 640 | 19,000 | 75 | 21 | 2.2 | 880 | 24,000 | 67 |
| -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||
| 50 µM Cu | 17,000 | 93 | 420 | 18,000 | 24 | 15 | 2.7 | 550 | 4400 | 25 | ||
| -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||
| 100 µM Cu | 30,000 | 170 | 410 | 15,000 | 79 | 21 | 2.3 | 610 | 13,000 | 40 | ||
| -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |||
| Quillay | Control | 16,200 | 12.2 | 158 | 19,600 | 52.6 | 7 | 1.6 | 1780 | 7620 | 42.8 | |
| [1790] | [2.9] | [40.9] | [1820] | [14] | [2.2] | [0.3] | [497] | [1160] | [7.4] | |||
| 50 µM Cu | 11,400 | 67.2 | 172 | 17,800 | 30.4 | 4.6 | 1.62 | 1840 | 4160 | 33 | ||
| [2040] | [14.5] | [59] | [3900] | [7] | [1] | [0.3] | [385] | [1080] | [7.4] | |||
| 100 µM Cu | 14,600 | 96.6 | 126 | 20,800 | 63 | 4.72 | 1.54 | 1820 | 5980 | 37.6 | ||
| [1340] | [23.1] | [34.3] | [2680] | [24.3] | [1.7] | [0.3] | [536] | [1350] | [8.1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milla-Moreno, E.; Guy, R.D.; Soolanayakanahally, R.Y. Enlightening the Pathway of Phytoremediation: Ecophysiology and X-ray Fluorescence Visualization of Two Chilean Hardwoods Exposed to Excess Copper. Toxics 2022, 10, 237. https://doi.org/10.3390/toxics10050237
Milla-Moreno E, Guy RD, Soolanayakanahally RY. Enlightening the Pathway of Phytoremediation: Ecophysiology and X-ray Fluorescence Visualization of Two Chilean Hardwoods Exposed to Excess Copper. Toxics. 2022; 10(5):237. https://doi.org/10.3390/toxics10050237
Chicago/Turabian StyleMilla-Moreno, Estefanía, Robert Dean Guy, and Raju Y. Soolanayakanahally. 2022. "Enlightening the Pathway of Phytoremediation: Ecophysiology and X-ray Fluorescence Visualization of Two Chilean Hardwoods Exposed to Excess Copper" Toxics 10, no. 5: 237. https://doi.org/10.3390/toxics10050237
APA StyleMilla-Moreno, E., Guy, R. D., & Soolanayakanahally, R. Y. (2022). Enlightening the Pathway of Phytoremediation: Ecophysiology and X-ray Fluorescence Visualization of Two Chilean Hardwoods Exposed to Excess Copper. Toxics, 10(5), 237. https://doi.org/10.3390/toxics10050237