Potential Role of Lipase Activity on the Internal Exposure Assessment of Glycidol Released from Its Fatty Acid Esters
Abstract
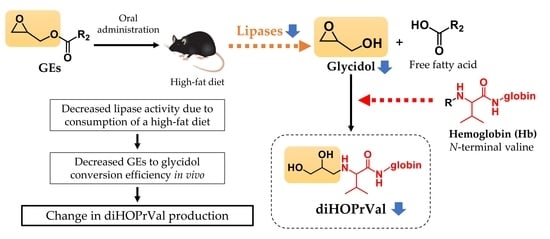
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Human Blood Sample Collection
2.3. Animals
2.4. Glycidol–Hemoglobin Adduct (diHOPrVal) Measurement
2.4.1. Human Blood Sample
2.4.2. Animal Blood Sample
2.5. Solid-Phase Extraction
2.6. Detection of Hb Adduct by LC–MS/MS
2.7. Measurement of Lipase Activity
2.8. Statistical Analysis
3. Results
3.1. Levels of diHOPrVal in the Blood of Humans Fed with Charcoal-Grilled Pork
3.2. Lipase Activity in Mice Fed High-Fat Diet
3.3. Relationship between Decreased Lipase Activity and the Release of Glycidol from GEs in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aniołowska, M.; Kita, A. Monitoring of glycidyl fatty acid esters in refined vegetable oils from retail outlets by LC–MS. J. Sci. Food Agric. 2016, 96, 4056–4061. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.; Dubois, M.; Scholz, G.; Seefelder, W.; Chuat, J.Y.; Schilter, B. Application of gastrointestinal modelling to the study of the digestion and transformation of dietary glycidyl esters. Food Addit. Contam. Part A 2013, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Aasa, J.; Vare, D.; Motwani, H.V.; Jenssen, D.; Törnqvist, M. Quantification of the mutagenic potency and repair of glycidol-induced DNA lesions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 805, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Fujii, K.; Sarada, M.; Saito, H.; Kawabata, M.; Naruse, K.; Yuki, K.; Nakagiri, H.; Honda, H.; Tamaki, Y.; et al. Genotoxicity studies of glycidol fatty acid ester (glycidol linoleate) and glycidol. Food Chem. Toxicol. 2012, 50, 3927–3933. [Google Scholar] [CrossRef]
- Inagaki, R.; Uchino, K.; Shimamura, Y.; Masuda, S. Investigation of DNA damage of glycidol and glycidol fatty acid esters using Fpg-modified comet assay. Fundam. Toxicol. Sci. 2019, 6, 9–14. [Google Scholar] [CrossRef]
- Canter, D.A.; Zeiger, E.; Haworth, S.; Lawlor, T.; Mortelmans, K.; Speck, W. Comparative mutagenicity of aliphatic epoxides in Salmonella. Mutat. Res. 1986, 172, 105–138. [Google Scholar] [CrossRef]
- Melnick, R.L. Carcinogenicity and mechanistic insights on the behavior of epoxides and epoxide-forming chemicals. Ann. N. Y. Acad. Sci. 2002, 982, 177–189. [Google Scholar] [CrossRef]
- Akane, H.; Shiraki, A.; Imatanaka, N.; Akahori, Y.; Itahashi, M.; Ohishi, T.; Mitsumori, K.; Shibutani, M. Glycidol induces axonopathy by adult-stage exposure and aberration of hippocampal neurogenesis affecting late-stage differentiation by developmental exposure in rats. Toxicol. Sci. 2013, 134, 140–154. [Google Scholar] [CrossRef]
- Aasa, J.; Abramsson-Zetterberg, L.; Carlsson, H.; Törnqvist, M. The genotoxic potency of glycidol established from micronucleus frequency and hemoglobin adduct levels in mice. Food Chem. Toxicol. 2017, 100, 168–174. [Google Scholar] [CrossRef]
- Honda, H.; Onishi, M.; Fujii, K.; Ikeda, N.; Yamaguchi, T.; Fujimori, T.; Nishiyama, N.; Kasamatsu, T. Measurement of glycidol hemoglobin adducts in humans who ingest edible oil containing small amounts of glycidol fatty acid esters. Food Chem. Toxicol. 2011, 49, 2536–2540. [Google Scholar] [CrossRef]
- Abraham, K.; Hielscher, J.; Kaufholz, T.; Mielke, H.; Lampen, A.; Monien, B. The hemoglobin adduct N-(2, 3-dihydroxypropyl)-valine as biomarker of dietary exposure to glycidyl esters: A controlled exposure study in humans. Arch. Toxicol. 2019, 93, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Hielscher, J.; Monien, B.H.; Abraham, K.; Jessel, S.; Seidel, A.; Lampen, A. An isotope-dilution UPLC–MS/MS technique for the human biomonitoring of the internal exposure to glycidol via a valine adduct at the N-terminus of hemoglobin. J. Chromatogr. B 2017, 1059, 7–13. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Dussort, P.; Günther, H.; Hanlon, P.; Honda, H.; Mally, A.; O’Hagan, S.; Scholz, G.; Seidel, A.; Swenberg, J.; et al. Exposure assessment of process-related contaminants in food by biomarker monitoring. Arch. Toxicol. 2018, 92, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Landin, H.H.; Osterman-Golkar, S.; Zorcec, V.; Törnqvist, M. Biomonitoring of epichlorohydrin by hemoglobin adducts. Anal. Biochem. 1996, 240, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Fujii, K.; Yamaguchi, T.; Ikeda, N.; Nishiyama, N.; Kasamatsu, T. Glycidol exposure evaluation of humans who have ingested diacylglycerol oil containing glycidol fatty acid esters using hemoglobin adducts. Food Chem. Toxicol. 2012, 50, 4163–4168. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Törnqvist, M.; Nishiyama, N.; Kasamatsu, T. Characterization of glycidol–hemoglobin adducts as biomarkers of exposure and in vivo dose. Toxicol. Appl. Pharmacol. 2014, 275, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, R.; Hirai, C.; Shimamura, Y.; Masuda, S. Formation of glycidol fatty acid esters in meat samples cooked by various methods. J. Food Process. Technol. 2016, 7, 2–7. [Google Scholar] [CrossRef]
- Shimamura, Y.; Inagaki, R.; Oike, M.; Dong, B.; Gong, W.; Masuda, S. Glycidol fatty acid ester and 3-monochloropropane-1, 2-diol fatty acid ester in commercially prepared foods. Foods 2021, 10, 2905. [Google Scholar] [CrossRef] [PubMed]
- Landin, H.H.; Tareke, E.; Rydberg, P.; Olsson, U.; Törnqvist, M. Heating of food and haemoglobin adducts from carcinogens: Possible precursor role of glycidol. Food Chem. Toxicol. 2000, 38, 963–969. [Google Scholar] [CrossRef]
- Mukherjee, M. Human digestive and metabolic lipases—A brief review. J. Mol. Catal. B Enzym. 2003, 22, 369–376. [Google Scholar] [CrossRef]
- Yang, R.; Le, G.; Li, A.; Zheng, J.; Shi, Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006, 22, 1185–1191. [Google Scholar] [CrossRef]
- Rydberg, P. The patent belongs to Adduct Analys AB, Enebyberg, Sweden. European Patent EP1738177, 11 February 2009. [Google Scholar]
- Rydberg, P.; von Stedingk, H.; Magnér, J.; Björklund, J. LC/MS/MS analysis of N-terminal protein adducts with improved sensitivity: A comparison of selected Edman isothiocyanate reagents. Int. J. Anal. Chem. 2009, 2009, 153472. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Moriwaki, J.; Shiiba, D.; Nohara, H.; Kudo, N.; Katsuragi, Y. Elimination of glycidyl palmitate in diolein by treatment with activated bleaching earth. J. Oleo Sci. 2012, 61, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Fujita, Y.; Hayashi, A.; Ikeda, N.; Ito, Y.; Morita, O. Genotoxicity evaluation of alpha-linolenic acid-diacylglycerol oil. Toxicol. Rep. 2016, 3, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.E.; Abraham, K.; Berger-Preiss, E.; Hansen, T.; Apel, E.; Schuchardt, S.; Vogt, C.; Bakhiya, N.; Creutzenberg, O.; Lampen, A. Relative oral bioavailability of glycidol from glycidyl fatty acid esters in rats. Arch. Toxicol. 2013, 87, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, Y.; Shiro, H.; Nakamura, S.; Kondo, N.; Jin, N.; Suzuki, N.; Ooi, N.; Kudo, N. A new analytical method for the quantification of glycidol fatty acid esters in edible oils. J. Oleo. Sci. 2010, 59, 81–88. [Google Scholar] [CrossRef]
- Schricker, B.R.; Miller, D.D.; Stouffer, J.R. Measurement and content of nonheme and total iron in muscle. J. Food Sci. 2006, 47, 740–743. [Google Scholar] [CrossRef]
- Knüttel-Gustavsen, S.; Harmeyer, J. The content of L-carnitine in meat after different methods of heat treatment. British Food J. 2011, 113, 1114–1126. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Amano, I.; Hirose, S.; Tsuruta, Y.; Hara, S.; Murata, M.; Imai, T. Effects of L-carnitine supplementation on renal anemia in poor responders to erythropoietin. Blood Purif. 2001, 19, 24–32. [Google Scholar] [CrossRef]
- Williams, L.; Davis, J.A.; Lowenthal, D.T. The influence of food on the absorption and metabolism of drugs. Med. Clin. N. Am. 1993, 77, 815–829. [Google Scholar] [CrossRef]
- Shimamura, Y.; Okuda, A.; Ichikawa, K.; Inagaki, R.; Ito, S.; Honda, H.; Masuda, S. Factors influencing the formation of chemical–hemoglobin adducts. Toxics 2021, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Kurata, Y.; Harada, T.; Tamaki, Y.; Nishiyama, N.; Kasamatsu, T. Species differences in toxicokinetic parameters of glycidol after a single dose of glycidol or glycidol linoleate in rats and monkeys. J. Toxicol. Sci. 2012, 37, 691–698. [Google Scholar] [CrossRef]
- Barchuk, M.; Nagasawa, T.; Murakami, M.; López, G.; Baldi, J.; Miksztowicz, V.; Rubio, M.; Schreier, L.; Nakajima, K.; Berg, G. The antagonic behavior of GPIHBP1 between EAT and circulation does not reflect lipolytic enzymes levels in the tissue and serum from coronary patients. Clin. Chim. Acta 2020, 510, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Minato, K.; Shiroya, Y.; Nakae, Y.; Kondo, T. The effect of chronic exercise on the rat pancreas. Int. J. Gastrointest. Cancer 2000, 27, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Inagaki, R.; Honda, H.; Masuda, S. Does external exposure of glycidol-related chemicals influence the forming of the hemoglobin adduct, N-(2, 3-dihydroxypropyl) valine, as a biomarker of internal exposure to glycidol? Toxics 2020, 8, 119. [Google Scholar] [CrossRef]
- Konturek, S.J.; Tasler, J.; Obtulowicz, W. Effect of exercise on gastrointestinal secretions. J. Appl. Physiol. 1973, 34, 324–328. [Google Scholar] [CrossRef]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimamura, Y.; Inagaki, R.; Oike, M.; Wada, Y.; Honda, H.; Masuda, S. Potential Role of Lipase Activity on the Internal Exposure Assessment of Glycidol Released from Its Fatty Acid Esters. Toxics 2023, 11, 175. https://doi.org/10.3390/toxics11020175
Shimamura Y, Inagaki R, Oike M, Wada Y, Honda H, Masuda S. Potential Role of Lipase Activity on the Internal Exposure Assessment of Glycidol Released from Its Fatty Acid Esters. Toxics. 2023; 11(2):175. https://doi.org/10.3390/toxics11020175
Chicago/Turabian StyleShimamura, Yuko, Ryo Inagaki, Minami Oike, Yuri Wada, Hiroshi Honda, and Shuichi Masuda. 2023. "Potential Role of Lipase Activity on the Internal Exposure Assessment of Glycidol Released from Its Fatty Acid Esters" Toxics 11, no. 2: 175. https://doi.org/10.3390/toxics11020175
APA StyleShimamura, Y., Inagaki, R., Oike, M., Wada, Y., Honda, H., & Masuda, S. (2023). Potential Role of Lipase Activity on the Internal Exposure Assessment of Glycidol Released from Its Fatty Acid Esters. Toxics, 11(2), 175. https://doi.org/10.3390/toxics11020175