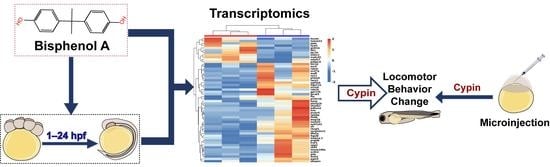
Stage-Related Neurotoxicity of BPA in the Development of Zebrafish Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents Preparation
2.2. BPA Exposure of Zebrafish Embryos
2.3. Locomotor Behavioral Analysis
2.4. Transcriptomics Analysis
2.5. Real-Time Quantitative PCR Analysis
2.6. Microinjection
2.7. Guanine Deaminase Activity Assay
2.8. Statistical Analysis
3. Results
3.1. Survival Rate of Zebrafish Exposed to BPA
3.2. Effects of BPA on Zebrafish Locomotor Behavior
3.3. Transcriptome Analysis of Zebrafish Larvae at Different Developmental Periods after BPA Exposure
3.4. Changes in Cypin during Zebrafish Embryonic Development
3.5. Effect of BPA on the Activity of Guanine Deaminase in Zebrafish Embryos during 1–24 hpf
3.6. Changes in Cypin Expression after Microinjection of Cypin mRNA
3.7. The Locomotor Behavior Change of Zebrafish Larvae after Microinjection of Cypin mRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Michalowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Impact of phthalates and bisphenols plasticizers on haemocyte immune function of aquatic invertebrates: A review on physiological, biochemical, and genomic aspects. J. Hazard. Mater. 2021, 419, 126426. [Google Scholar] [CrossRef]
- Takeuchi, T.; Tsutsumi, O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 2002, 291, 76–78. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Kannan, K. Blood and urinary bisphenol A concentrations in children, adults, and pregnant women from china: Partitioning between blood and urine and maternal and fetal cord blood. Environ. Sci. Technol. 2013, 47, 4686–4694. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Ciência Saúde Coletiva 2012, 17, 407–434. [Google Scholar] [CrossRef]
- Gounden, V.; Warasally, M.Z.; Magwai, T.; Naidoo, R.; Chuturgoon, A. A pilot study: Bisphenol-A and Bisphenol-A glucuronide levels in mother and child pairs in a South African population. Reprod. Toxicol. 2019, 89, 93–99. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef]
- Xu, X.; Hong, X.; Xie, L.; Li, T.; Yang, Y.; Zhang, Q.; Zhang, G.; Liu, X. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Horm. Behav. 2012, 62, 480–490. [Google Scholar] [CrossRef]
- Jasarevic, E.; Williams, S.A.; Vandas, G.M.; Ellersieck, M.R.; Liao, C.; Kannan, K.; Roberts, R.M.; Geary, D.C.; Rosenfeld, C.S. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm. Behav. 2013, 63, 180–189. [Google Scholar] [CrossRef]
- Lee, B.E.; Park, H.; Hong, Y.C.; Ha, M.; Kim, Y.; Chang, N.; Kim, B.N.; Kim, Y.J.; Yu, S.D.; Ha, E.H. Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children’s Environmental Health) study. Int. J. Hyg. Environ. Health 2014, 217, 328–334. [Google Scholar] [CrossRef]
- Panzica-Kelly, J.M.; Zhang, C.X.; Augustine-Rauch, K.A. Optimization and Performance Assessment of the Chorion-Off [Dechorinated] Zebrafish Developmental Toxicity Assay. Toxicol. Sci. 2015, 146, 127–134. [Google Scholar] [CrossRef]
- To, K.T.; St Mary, L.; Wooley, A.H.; Wilbanks, M.S.; Bednar, A.J.; Perkins, E.J.; Truong, L.; Tanguay, R.L.; Garcia-Reyero, N. Morphological and Behavioral Effects in Zebrafish Embryos after Exposure to Smoke Dyes. Toxics 2021, 9, 9. [Google Scholar] [CrossRef]
- Tran, C.M.; Do, T.N.; Kim, K.T. Comparative Analysis of Neurotoxicity of Six Phthalates in Zebrafish Embryos. Toxics 2021, 9, 5. [Google Scholar] [CrossRef]
- Parker, M.O.; Brock, A.J.; Walton, R.T.; Brennan, C.H. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front. Neural Circuits 2013, 7, 63. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Itoh, M.; Kim, C.H.; Palardy, G.; Oda, T.; Jiang, Y.J.; Maust, D.; Yeo, S.Y.; Lorick, K.; Wright, G.J.; Ariza-McNaughton, L.; et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 2003, 4, 67–82. [Google Scholar] [CrossRef]
- Faustino, A.I.; Tacão-Monteiro, A.; Oliveira, R.F. Mechanisms of social buffering of fear in zebrafish. Sci. Rep. 2017, 7, 44329. [Google Scholar] [CrossRef] [Green Version]
- Golzio, C.; Willer, J.; Talkowski, M.E.; Oh, E.C.; Taniguchi, Y.; Jacquemont, S.; Reymond, A.; Sun, M.; Sawa, A.; Gusella, J.F.; et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 2012, 485, 363–367. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- de Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing: A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef]
- Buss, R.R.; Drapeau, P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J. Neurophysiol. 2001, 86, 197–210. [Google Scholar] [CrossRef]
- Stehr, C.M.; Linbo, T.L.; Incardona, J.P.; Scholz, N.L. The developmental neurotoxicity of fipronil: Notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol. Sci. 2006, 92, 270–278. [Google Scholar] [CrossRef]
- Costa-Pinto, F.A.; Cohn, D.W.; Sa-Rocha, V.M.; Sa-Rocha, L.C.; Palermo-Neto, J. Behavior: A relevant tool for brain-immune system interaction studies. Ann. N. Y. Acad. Sci. 2009, 1153, 107–119. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.; Hooyberghs, J.; De Coen, W.; Witters, H.E. Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotoxicol. Teratol. 2010, 32, 460–471. [Google Scholar] [CrossRef]
- Chen, J.; Liu, N.; Zhang, H.; Zhao, Y.; Cao, X. The effects of Aeromonas hydrophila infection on oxidative stress, nonspecific immunity, autophagy, and apoptosis in the common carp. Dev. Comp. Immunol. 2020, 105, 103587. [Google Scholar] [CrossRef]
- Rotimi, O.A.; Olawole, T.D.; De Campos, O.C.; Adelani, I.B.; Rotimi, S.O. Bisphenol A in Africa: A review of environmental and biological levels. Sci. Total Environ. 2021, 764, 142854. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.C.; Leonard, L.S.; Maness, S.C.; Wagner, B.L.; Conner, K.; Zacharewski, T.; Safe, S.; McDonnell, D.P.; Gaido, K.W. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998, 142, 203–214. [Google Scholar] [CrossRef]
- Burkhardt, F.; Schulz, S.D.; Hellwig, E.; Vach, K.; Tomakidi, P.; Polydorou, O. Low-dose Bisphenol A and its analogues Bisphenol F and S activate estrogen receptor ss and slightly modulate genes in human gingival keratinocytes. Dent. Mater. 2021, 37, 625–635. [Google Scholar] [CrossRef]
- Sang, C.; Song, Y.; Jin, T.W.; Zhang, S.; Fu, L.; Zhao, Y.; Zou, X.; Wang, Z.; Gao, H.; Liu, S. Bisphenol A induces ovarian cancer cell proliferation and metastasis through estrogen receptor-alpha pathways. Environ. Sci. Pollut. Res. Int. 2021, 28, 36060–36068. [Google Scholar] [CrossRef]
- Ejaredar, M.; Lee, Y.; Roberts, D.J.; Sauve, R.; Dewey, D. Bisphenol A exposure and children’s behavior: A systematic review. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 175–183. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L.; Kwiatkowski, C.F. Prenatal exposure to bisphenol A and hyperactivity in children: A systematic review and meta-analysis. Environ. Int. 2018, 114, 343–356. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Warga, R.M.; Schilling, T.F. Origin and organization of the zebrafish fate map. Development 1990, 108, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Hanneman, E.; Westerfield, M. Early expression of acetylcholinesterase activity in functionally distinct neurons of the zebrafish. J. Comp. Neurol. 1989, 284, 350–361. [Google Scholar] [CrossRef]
- Wilson, S.W.; Ross, L.S.; Parrett, T.; Easter, S.S., Jr. The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development 1990, 108, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Billat, P.A.; Brochot, C.; Brion, F.; Beaudouin, R. A PBPK model to evaluate zebrafish eleutheroembryos’ actual exposure: Bisphenol A and analogs’ (AF, F, and S) case studies. Environ. Sci. Pollut. Res. Int. 2022, 30, 7640–7653. [Google Scholar] [CrossRef]
- Firestein, B.L.; Firestein, B.L.; Brenman, J.E.; Aoki, C.; Sanchez-Perez, A.M.; El-Husseini, A.E.; Bredt, D.S. Cypin: A cytosolic regulator of PSD-95 postsynaptic targeting. Neuron 1999, 24, 659–672. [Google Scholar] [CrossRef]
- Paletzki, R.F. Cloning and characterization of guanine deaminase from mouse and rat brain. Neuroscience 2002, 109, 15–26. [Google Scholar] [CrossRef]
- Akum, B.F.; Chen, M.; Gunderson, S.I.; Riefler, G.M.; Scerri-Hansen, M.M.; Firestein, B.L. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat. Neurosci. 2004, 7, 145–152. [Google Scholar] [CrossRef]
- Fernandez, J.R.; Welsh, W.J.; Firestein, B.L. Structural characterization of the zinc binding domain in cytosolic PSD-95 interactor (cypin): Role of zinc binding in guanine deamination and dendrite branching. Proteins 2008, 70, 873–881. [Google Scholar] [CrossRef]
- Patel, M.V.; Swiatkowski, P.; Kwon, M.; Rodriguez, A.R.; Campagno, K.; Firestein, B.L. A Novel Short Isoform of Cytosolic PSD-95 Interactor (Cypin) Regulates Neuronal Development. Mol. Neurobiol. 2018, 55, 6269–6281. [Google Scholar] [CrossRef]
- Braunschweig, D.; Krakowiak, P.; Duncanson, P.; Boyce, R.; Hansen, R.L.; Ashwood, P.; Hertz-Picciotto, I.; Pessah, I.N.; Van de Water, J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl. Psychiatry 2013, 3, e277. [Google Scholar] [CrossRef] [PubMed]
- Schretlen, D.J.; Harris, J.C.; Park, K.S.; Jinnah, H.A.; del Pozo, N.O. Neurocognitive functioning in Lesch-Nyhan disease and partial hypoxanthine-guanine phosphoribosyltransferase deficiency. J. Int. Neuropsychol. Soc. 2001, 7, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Ogungbemi, A.O.; Teixido, E.; Massei, R.; Scholz, S.; Kuster, E. Automated measurement of the spontaneous tail coiling of zebrafish embryos as a sensitive behavior endpoint using a workflow in KNIME. MethodsX 2021, 8, 101330. [Google Scholar] [CrossRef]
- Yu, H.; Ma, L.; Liu, D.; Wang, Y.; Pei, X.; Duan, Z.; Ma, M.; Zhang, Y. Involvement of NMDAR/PSD-95/nNOS-NO-cGMP pathway in embryonic exposure to BPA induced learning and memory dysfunction of rats. Environ. Pollut. 2020, 266, 115055. [Google Scholar] [CrossRef]
- Charych, E.I.; Akum, B.F.; Goldberg, J.S.; Jornsten, R.J.; Rongo, C.; Zheng, J.Q.; Firestein, B.L. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J. Neurosci. 2006, 26, 10164–10176. [Google Scholar] [CrossRef]
- Wong, W.T.; Wong, R.O. Rapid dendritic movements during synapse formation and rearrangement. Curr. Opin. Neurobiol. 2000, 10, 118–124. [Google Scholar] [CrossRef]
- Chen, H.; Firestein, B.L. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J. Neurosci. 2007, 27, 8378–8386. [Google Scholar] [CrossRef] [PubMed] [Green Version]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Kong, W.; Liu, Y.; Ma, Q.; Shao, Q.; Zeng, L.; Chao, Y.; Song, X.; Zhang, J. Stage-Related Neurotoxicity of BPA in the Development of Zebrafish Embryos. Toxics 2023, 11, 177. https://doi.org/10.3390/toxics11020177
Liu J, Kong W, Liu Y, Ma Q, Shao Q, Zeng L, Chao Y, Song X, Zhang J. Stage-Related Neurotoxicity of BPA in the Development of Zebrafish Embryos. Toxics. 2023; 11(2):177. https://doi.org/10.3390/toxics11020177
Chicago/Turabian StyleLiu, Jianjun, Wenyu Kong, Yuchen Liu, Qiyao Ma, Qi Shao, Liwen Zeng, Yu Chao, Xiaoyao Song, and Jie Zhang. 2023. "Stage-Related Neurotoxicity of BPA in the Development of Zebrafish Embryos" Toxics 11, no. 2: 177. https://doi.org/10.3390/toxics11020177
APA StyleLiu, J., Kong, W., Liu, Y., Ma, Q., Shao, Q., Zeng, L., Chao, Y., Song, X., & Zhang, J. (2023). Stage-Related Neurotoxicity of BPA in the Development of Zebrafish Embryos. Toxics, 11(2), 177. https://doi.org/10.3390/toxics11020177