Unveiling Molecular Effects of the Secondary Metabolite 2-Dodecanone in the Model Hymenopteran Nasonia vitripennis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wasp Population
2.2. Exposure Conditions, Short and Long-Term Assays
2.3. RNA Extraction and cDNA Synthesis
2.4. Quantitative Real-Time PCR
2.5. Ecdysteroid Titration
2.6. Energy Reserves
2.7. Alkaline Comet Assay
2.8. Female Egg-Laying Test
2.9. Data Analysis
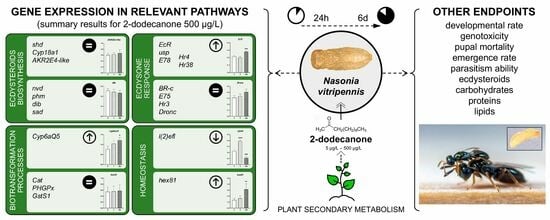
3. Results
3.1. Acute Toxicity of 2-Dodecanone
3.1.1. Gene Expression Analysis
Ecdysone Biosynthesis Pathway
Ecdysone-Signalling Pathway
Detoxification- and Homeostasis-Related Biomarkers
3.1.2. Impact on Energy Reserves and Potential Genotoxicity
3.2. Long-Term Exposure to 2-Dodecanone
3.2.1. Time Development and Emergence Rate
3.2.2. Impact on Female Reproduction and Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.Z.; Law, F.C.P. Adverse effects of pesticides and related chemicals on enzyme and hormone systems of fish, amphibians and reptiles: A review. Proc. Pak. Acad. Sci. 2005, 42, 315–323. [Google Scholar]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Amsterdam, Netherlands, 2011; pp. 438–453. [Google Scholar]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.; Pereira, E.J.; Aguiar, R.W.; Oliveira, E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Forney, F.W.; Markovetz, A.J. The biology of methyl ketones. J. Lipid Res. 1971, 12, 383–395. [Google Scholar] [CrossRef]
- Williams, W.G.; Kennedy, G.G.; Yamamoto, R.T.; Thacker, J.D.; Bordner, J. 2-Tridecanone: A Naturally Occurring Insecticide from the Wild Tomato Lycopersicon hirsutum f. glabratum. Science 1980, 207, 888–889. [Google Scholar] [CrossRef]
- Antonious, G.F.; Snyder, J.C. Repellency and oviposition deterrence of wild tomato leaf extracts to spider mites, Tetranychus urticae Koch. J. Environ. Sci. Health B 2015, 50, 667–673. [Google Scholar]
- Ndungu, M.; Lwande, W.; Hassanali, A.; Moreka, L.; Chhabra, S.C. Cleome monophylla essential oil and its constituents as tick (Rhipicephalus appendiculatus) and maize weevil (Sitophilus zeamais) repellents. Entomol. Exp. Et Appl. 1995, 76, 217–222. [Google Scholar] [CrossRef]
- Kennedy, G.G. 2-tridecanone, tomatoes and Heliothis zea: Potential incompatibility of plant antibiosis with insecticidal control. Entomol. Exp. Et Appl. 1984, 35, 305–311. [Google Scholar] [CrossRef]
- Gonçalves, M.I.F.; Maluf, W.R.; Gomes, L.A.A.; Barbosa, L.V. Variation of 2-Tridecanone level in tomato plant leaflets and resistance to two mite species (Tetranychus sp.). Euphytica 1998, 104, 33–38. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Witting-Bissinger, B.E.; Stumpf, C.F.; Donohue, K.V.; Apperson, C.S.; Roe, R.M. Novel arthropod repellent, BioUD, is an efficacious alternative to deet. J. Med. Entomol. 2008, 45, 891–898. [Google Scholar] [CrossRef]
- Bohbot, J.D.; Dickens, J.C. Insect repellents: Modulators of mosquito odorant receptor activity. PLoS ONE 2010, 5, e12138. [Google Scholar] [CrossRef]
- Zhu, J.; Dhammi, A.; van Kretschmar, J.B.; Vargo, E.L.; Apperson, C.S.; Michael Roe, R. Novel use of aliphatic n-methyl ketones as a fumigant and alternative to methyl bromide for insect control. Pest Manag. Sci. 2018, 74, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Y.; Xiang, M.; Shang, Q.; Gao, X. The retardant effect of 2-Tridecanone, mediated by Cytochrome P450, on the Development of Cotton bollworm, Helicoverpa armigera. BMC Genom. 2016, 17, 954. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [PubMed]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Ventrella, E.; Adamski, Z.; Chudzińska, E.; Miądowicz-Kobielska, M.; Marciniak, P.; Büyükgüzel, E.; Büyükgüzel, K.; Erdem, M.; Falabella, P.; Scrano, L.; et al. Solanum tuberosum and Lycopersicon esculentum Leaf Extracts and Single Metabolites Affect Development and Reproduction of Drosophila melanogaster. PLoS ONE 2016, 11, e0155958. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Keefer, D.A.; Ergin, E.; Uçkan, F. Morphology and Ultrastructure of Brain Tissue and Fat Body from the Flesh Fly, Sarcophaga bullata Parker (Diptera: Sarcophagidae), Envenomated by the Ectoparasitic Wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae). Psyche 2011, 2011, 520875. [Google Scholar]
- Werren, J.H.; Richards, S.; Desjardins, C.A.; Niehuis, O.; Gadau, J.; Colbourne, J.K.; The Nasonia Genome Working Group; Beukeboom, L.W.; Desplan, C.; Elsik, G.C.; et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 2010, 327, 343–348, Correction in Science 2010, 327, 1577. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, P.R.; Cook, N.; Blackburn, C.V.; Gill, S.M.; Green, J.; Shuker, D.M. Sex allocation theory reveals a hidden cost of neonicotinoid exposure in a parasitoid wasp. Proc. Biol. Sci. 2015, 282, 20150389. [Google Scholar] [CrossRef] [PubMed]
- Tappert, L.; Pokorny, T.; Hofferberth, J.; Ruther, J. Sublethal doses of imidacloprid disrupt sexual communication and host finding in a parasitoid wasp. Sci. Rep. 2017, 7, 42756. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Loehlin, D.W. The parasitoid wasp Nasonia: An emerging model system with haploid male genetics. Cold Spring Harb. Protoc. 2009, 2009, pdb.emo134. [Google Scholar] [CrossRef] [PubMed]
- Chirault, M.; Lucas, C.; Goubault, M.; Chevrier, C.; Bressac, C.; Lécureuil, C. A combined approach to heat stress effect on male fertility in Nasonia vitripennis: From the physiological consequences on spermatogenesis to the reproductive adjustment of females mated with stressed males. PLoS ONE 2015, 10, e0120656. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Park, S.Y.; Choi, J. Expression of catalase and glutathione S-transferase genes in Chironomus riparius on exposure to cadmium and nonylphenol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 399–408. [Google Scholar] [CrossRef]
- Nair, P.M.; Choi, J. Modulation in the mRNA expression of ecdysone receptor gene in aquatic midge, Chironomus riparius upon exposure to nonylphenol and silver nanoparticles. Environ. Toxicol. Pharmacol. 2012, 33, 98–106. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Porcheron, P.; Moriniere, M.; Grassi, J.; Pradelles, P. Development of an enzyme immunoassay for ecdysteroids using acetylcholinesterase as label. Insect Biochem. 1989, 19, 117–122. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 302–304. [Google Scholar]
- Van Handel, E. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 299–301. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 14 February 2024).
- Amichot, M.; Curty, C.; Benguettat-Magliano, O.; Gallet, A.; Wajnberg, E. Side effects of Bacillus thuringiensis var. kurstaki on the hymenopterous parasitic wasp Trichogramma chilonis. Environ. Sci. Pollut. Res. Int. 2016, 23, 3097–3103. [Google Scholar] [CrossRef]
- Riddiford, L.M.; Cherbas, P.; Truman, J.W. Ecdysone receptors and their biological actions. Vitam. Horm. 2000, 60, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Spindler, K.D.; Hönl, C.; Tremmel, C.; Braun, S.; Ruff, H.; Spindler-Barth, M. Ecdysteroid hormone action. Cell. Mol. Life Sci. 2009, 66, 3837–3850. [Google Scholar] [CrossRef]
- Huet, F.; Ruiz, C.; Richards, G. Sequential gene activation by ecdysone in Drosophila melanogaster: The hierarchical equivalence of early and early late genes. Development 1995, 121, 1195–1204. [Google Scholar] [CrossRef]
- Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. The UV filter benzophenone 3 (BP-3) activates hormonal genes mimicking the action of ecdysone and alters embryo development in the insect Chironomus riparius (Diptera). Environ. Pollut. 2014, 192, 19–26. [Google Scholar] [CrossRef]
- Planelló, R.; Martínez-Guitarte, J.L.; Morcillo, G. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius. Chemosphere 2008, 71, 1870–1876. [Google Scholar] [CrossRef]
- Planelló, R.; Herrero, O.; Martínez-Guitarte, J.L.; Morcillo, G. Comparative effects of butyl benzyl phthalate (BBP) and di(2-ethylhexyl) phthalate (DEHP) on the aquatic larvae of Chironomus riparius based on gene expression assays related to the endocrine system, the stress response and ribosomes. Aquat. Toxicol. 2011, 105, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Planelló, R.; Servia, M.J.; Gómez-Sande, P.; Herrero, Ó.; Cobo, F.; Morcillo, G. Transcriptional responses, metabolic activity and mouthpart deformities in natural populations of Chironomus riparius larvae exposed to environmental pollutants. Environ. Toxicol. 2015, 30, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Martínez-Paz, P.; Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. DNA damage and transcriptional changes induced by tributyltin (TBT) after short in vivo exposures of Chironomus riparius (Diptera) larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. Effects of in vivo exposure to UV filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on endocrine signaling genes in the insect Chironomus riparius. Sci. Total Environ. 2013, 456–457, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, M.; Sánchez-Argüello, P.; Martínez-Guitarte, J.L. Vinclozolin alters the expression of hormonal and stress genes in the midge Chironomus riparius. Aquat. Toxicol. 2016, 174, 179–187. [Google Scholar] [CrossRef]
- de la Fuente, M.; Folgar, R.M.; Martínez-Paz, P.; Cortés, E.; Martínez-Guitarte, J.L.; Morales, M. Effect of environmental stressors on the mRNA expression of ecdysone cascade genes in Chironomus riparius. Environ. Sci. Pollut. Res. Int. 2022, 29, 10210–10221. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Herrero, Ó.; Planelló, R.; Morcillo, G. The plasticizer benzyl butyl phthalate (BBP) alters the ecdysone hormone pathway, the cellular response to stress, the energy metabolism, and several detoxication mechanisms in Chironomus riparius larvae. Chemsphere 2015, 128, 266–277. [Google Scholar] [CrossRef]
- Herrero, Ó.; Aquilino, M.; Sánchez-Argüello, P.; Planelló, R. The BPA-substitute bisphenol S alters the transcription of genes related to endocrine, stress response and biotransformation pathways in the aquatic midge Chironomus riparius (Diptera, Chironomidae). PLoS ONE 2018, 13, e0193387. [Google Scholar] [CrossRef] [PubMed]
- Arambourou, H.; Planelló, R.; Llorente, L.; Fuertes, I.; Barata, C.; Delorme, N.; Noury, P.; Herrero, Ó.; Villeneuve, A.; Bonnineau, C. Chironomus riparius exposure to field-collected contaminated sediments: From subcellular effect to whole-organism response. Sci. Total Environ. 2019, 671, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Planelló, R.; Llorente, L.; Herrero, Ó.; Novo, M.; Blanco-Sánchez, L.; Díaz-Pendón, J.A.; Fernández-Muñoz, R.; Ferrero, V.; de la Peña, E. Transcriptome analysis of aphids exposed to glandular trichomes in tomato reveals stress and starvation related responses. Sci. Rep. 2022, 12, 20154. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Rana, S.; Sarkar, S.; Smith, B.; Basu, P. Pesticide-induced oxidative stress in laboratory and field populations of native honeybees along intensive agricultural landscapes in two Eastern Indian states. Apidol 2015, 46, 107–129. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Belzunces, L.P.; Carvalho, G.A.; Brunet, J.L.; Badiou-Beneteau, A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 2013, 32, 2117–2124. [Google Scholar] [CrossRef]
- Mamidala, P.; Jones, S.C.; Mittapalli, O. Metabolic Resistance in Bed Bugs. Insects 2011, 2, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Landa, V.; Šula, J.; Marec, F.; Maťha, V.; Soldán, T. Methods for assessing exposure of insects. In Methods for Assessing Exposure of Human and Non-Human Biota; Tardiff, R.G., Goldstein, B., Eds.; John Wiley & Sons Ltd.: Chichester, NY, USA, 1991; pp. 249–266. [Google Scholar]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect cytochrome P450. In Comprehensive Molecular Insect Science; Gilbert, L.I., Latrou, K., Gill, S.S., Eds.; Elsevier: Oxford, UK, 2015; pp. 1–77. [Google Scholar]
- Oakeshott, J.G.; Johnson, R.M.; Berenbaum, M.R.; Ranson, H.; Cristino, A.S.; Claudianos, C. Metabolic enzymes associated with xenobiotic and chemosensory responses in Nasonia vitripennis. Insect Mol. Biol. 2010, 19, 147–163. [Google Scholar] [CrossRef]
- Nagamanju, P.; Hansen, I.A.; Burmester, T.; Meyer, S.R.; Scheller, K.; Dutta-Gupta, A. Complete sequence, expression and evolution of two members of the hexamerin protein family during the larval development of the rice moth, Corcyra cephalonica. Insect Biochem. Mol. Biol. 2003, 33, 73–80. [Google Scholar] [CrossRef]
- Cristino, A.S.; Nunes, F.M.; Barchuk, A.R.; Aguiar-Coelho, V.M.; Simões, Z.L.; Bitondi, M. Organization, evolution and transcriptional profile of hexamerin genes of the parasitic wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). Insect Mol. Biol. 2010, 19 (Suppl. S1), 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.E.; Buck, N.A. Storage proteins in ants during development and colony founding. J. Insect Physiol. 1995, 41, 885–894. [Google Scholar] [CrossRef]
- Zhao, L.; Pridgeon, J.W.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. Identification of Genes Differentially Expressed During Heat Shock Treatment in Aedes aegypti. J. Med. Entomol. 2009, 46, 490–495. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Coissac, E.; Melodelima, C.; Poupardin, R.; Riaz, M.; Chandor-Proust, A.; Reynaud, S. Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology. BMC Genom. 2010, 11, 216. [Google Scholar] [CrossRef]
- Yang, W.J.; Xu, K.K.; Cao, Y.; Meng, Y.L.; Liu, Y.; Li, C. Identification and Expression Analysis of Four Small Heat Shock Protein Genes in Cigarette Beetle, Lasioderma serricorne (Fabricius). Insects 2019, 10, 139. [Google Scholar] [CrossRef]
- Mason, P.J.; Hall, L.M.C.; Gausz, J. The expression of heat shock genes during normal development in Drosophila melanogaster (heat shock/abundant transcripts/developmental regulation). Molec. Gen. Genom. 1984, 194, 73–78. [Google Scholar] [CrossRef]
- Sonoda, S.; Ashfaq, M.; Tsumuki, H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch. Insect Biochem. Physiol. 2006, 62, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Wang, C.Z.; Kang, L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J. Insect Physiol. 2009, 55, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, L.X.; Shen, Y.; Huang, L.H.; Feng, Q.L. Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol. Biol. 2012, 21, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Guo, X.; Li, Y.; Gao, H.; Guo, X.; Xu, B. sHsp22.6, an intronless small heat shock protein gene, is involved in stress defence and development in Apis cerana cerana. Insect Biochem. Mol. Biol. 2014, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Mahdi, A.A.; Deo, S.; Ahmad, M.K.; Kumar, D. Assessment of genotoxicity and oxidative stress in pregnant women contaminated to organochlorine pesticides and its correlation with pregnancy outcome. Environ. Res. 2022, 204 Pt B, 112010. [Google Scholar] [CrossRef]
- Bull, S.; Fletcher, K.; Boobis, A.R.; Battershill, J.M. Evidence for genotoxicity of pesticides in pesticide applicators: A review. Mutagenesis 2006, 21, 93–103. [Google Scholar] [CrossRef]
- Singh, N.P. The comet assay: Reflections on its development, evolution and applications. Rev. Mutat. Res. 2016, 767, 23–30. [Google Scholar] [CrossRef]
- Brendler-Schwaab, S.; Hartmann, A.; Pfuhler, S.; Speit, G. The in vivo comet assay: Use and status in genotoxicity testing. Mutagenesis 2005, 20, 245–254. [Google Scholar] [CrossRef] [PubMed]
- McKelvey-Martin, V.J.; Green, M.H.L.; Schmezer, P.; Pool-Zobel, B.L.; De Méo, M.P.; Collins, A. The single cell gel electrophoresis assay (comet assay): A European review. Mutat. Res. 1993, 288, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef] [PubMed]
- Gastelbondo-Pastrana, B.I.; Fernandes, F.H.; Salvadori, D.M.F.; Santos, D.C.D. The comet assay in Ceraeochrysa claveri (Neuroptera: Chrysopidae): A suitable approach for detecting somatic and germ cell genotoxicity induced by agrochemicals. Chemsphere 2019, 235, 70–75. [Google Scholar] [CrossRef]
- Bastos, C.S.; de Almeida, R.P.; Suinaga, F.A. Selectivity of pesticides used on cotton (Gossypium hirsutum) to Trichogramma pretiosum reared on two laboratory-reared hosts. Pest Manag. Sci. 2006, 62, 91–98. [Google Scholar] [CrossRef]
- Saber, M.; Hejazi, M.J.; Kamali, K.; Moharramipour, S. Lethal and sublethal effects of fenitrothion and deltamethrin residues on the egg parasitoid Trissolcus grandis (Hymenoptera: Scelionidae). J. Econ. Entomol. 2005, 98, 35–40. [Google Scholar] [CrossRef]





| Gene | Primer Sequence (5′-3′) | Gene Reference | |
|---|---|---|---|
| ECDYSONE BIOSYNTHESIS PATHWAY | nvd | F GCCTGGATACGAGATTTAACG R CCAACCATTCGGGTAAACTG | NASONIABASE: NV17010 |
| phtm | F TCAAGTTTCCCAGTCCCAAG R ACGATGAAGACGACGAGCTT | XM_032601767.1 NASONIABASE: NV19009 | |
| dib | F CTTTATGCCGGAGCGATG R GCGACACATCCTGAGCAGT | XM_001601625.6 NASONIABASE: NV14828 | |
| sad | F GCCGGTGACACTACAGCTTAC R TAGAGCCGTAGCGACTCCTT | XM_008214583 NASONIABASE: NV11849 | |
| akr2e4 | F GCCGAAGGTAGACGAAATCA R GGCCAATTTGACAGCATTTT | XM_001603888.5 NASONIABASE: NV13784 | |
| shd | F GCCTCGAGAGATGCCTTCTA R AACTGCTTCGATGCTCTCGT | NM_001172547.1 NASONIABASE: NV17940 | |
| Cyp18a1 | F ACGACTCGCGAGGTCAATCT R GAAGTACTCGGGCTTGTGGA | XM_001600748.5 NASONIABASE: NV19010 | |
| ECDYSONE SIGNALING PATHWAY | EcR | F GCCCAGAAGGAGAAGGACAAA R CGTTGAGCGAGCCTATGGAG | NM_001159357.1 NASONIABASE: NV11088 |
| usp | F GTCCTGGGTCTTTAAACCTTG R CAGAGATGTTTGCTGCCAGA | XM_001605769.6 NASONIABASE: NV15186 | |
| E75 | F CGACACTGGCAACCTGACTG R GCACCGCATCACGACTCAT | NASONIABASE: NV50220 | |
| BR-C | F AGCTTCTCAAGAGCA R CTACTGAGAGAGCGCTGGTG | XM_008211399.2 NASONIABASE: NV15002 | |
| E78 | F CTCGACTCAGAACGCGATGA R CCTGGTGGACTCGTCGGTAA | XM_016987036.1 NASONIABASE: NV13864 | |
| Hr3 | F AAGCAGGAGACGACGACCAC R CGCAAGGGAGGACATACTGG | XM_016986225.1 NASONIABASE: NV50123 | |
| Hr4 | F GGAGGGTGAAACTGAGGATGG R ACTTCGGGCAATCTGACGAG | XM_008206002.2 NASONIABASE: NV14578 | |
| Hr38 | F GACGATACGCAGGGAGGAGA R CGAGCTTGCACATGGAGATG | HR3. XM_001601528.4 NASONIABASE: NV14578 | |
| Hr39 | F GATCTCAAGTCCCATCGCCA R TCGAGGCGTCCGAGTTATTG | XM_001603476.5 NASONIABASE: NV13389 | |
| Dronc | F GAAACTGAAATCCAAGGCATCG R GGCAAATCGTGAAACAACAGC | XM_032598125.1 NASONIABASE: NV15018 | |
| BIOTRANSFORMATION PROCESSES AND HOMEOSTASIS | Cyp6aQ5 | F GGAAATCGACGAAAAAGTTGG R TTTGCAGGTAACGCATGAAA | NM_001172525.1 NASONIABASE: NV13220 |
| Cat | F CGTGATCTTCGTGGTTTTGCTG R GGATTGGATCGCGGATGAAG | [30] | |
| PHGPx | F AAGTGTGGTTACACAGCTAAGCATT R GATATCCAAATTGATTACACGGAAA | [31] | |
| GstS1 | F GTGTGACCAGCAACGAATGG R TCCAGATCCTCCCATGTGCT | From Prodiamesa olivacea (Diptera; data unpublished) | |
| Ie2ef1 | F TCGAAGGAAAGCACG R GTGATGCTGAGGACT | XM_032597114.1 NASONIABASE: Nv13797 | |
| hex81 | F CAACAAGGAAGCAGT R GTGTCGAAGTCCTTG | NASONIABASE: NV12472 | |
| REFERENCE GENES | RpL6 | F AAGAAGACACCCAAGAAGGAA R ACAATGGGATCTGAGGTAGGA | NM_001159919.1 NASONIABASE: NV12167 |
| RpL7 | F AAGAAAGTCGAGCCCAAGAAG R GGCTGAATATCCTCGGCAAT | NASONIABASE: NV14954 | |
| EF-1a | F CACTTGATCTACAAATGCGGTG R CCTTCAGTTTGTCCAAGACC | NM_001172756.1 NASONIABASE: NV13182 |
| Control Acetone (0.05%) | 2-Dodecanone 5 µg/L | 2-Dodecanone 500 µg/L | |
|---|---|---|---|
| Pupal survival rate (%) (48 h) | 97.9 ± 2.9 (4 exp, 114 ind) | 96.9± 2.9 (4 exp, 107 ind) | 95.3 ± 0.7 (4 exp, 114 ind) |
| Ecdysteroids (fmol/mg) | 550 ± 124 (3 exp, 12 ind) | 653 ± 92 (3 exp, 12 ind) | 704 ± 177 (3 exp, 12 ind) |
| Proteins (µg/mg/individual) | 6.2 ± 0.7 (3 exp, 12 ind) | 6.1 ± 0.8 (3 exp, 12 ind) | 6.3 ± 0.7 (3 exp, 12 ind) |
| Lipids (µg/mg individual) | 13.7 ± 3.8 (3 exp, 12 ind) | 11.4 ± 2.3 (3 exp, 12 ind) | 14.0 ± 4.8 (3 exp, 12 ind) |
| Carbohydrates (µg/mg/individual) | 2.2 ± 1.3 (3 exp, 12 ind) | 2.2 ± 0.4 (3 exp, 12 ind) | 2.1 ± 0.9 (3 exp, 12 ind) |
| Comet assay | 155 ± 57 (10 exp, 30 ind) | - | 133 ± 56 (10 exp, 30 ind) |
| Emergence rate (%) (120 h) | 57.1% ± 1.8 (5 exp, 105 ind) | - | 49.2% ± 2.1 (5 exp, 120 ind) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Planelló, R.; Aquilino, M.; Beaugeard, L.; Llorente, L.; Herrero, Ó.; Siaussat, D.; Lécureuil, C. Unveiling Molecular Effects of the Secondary Metabolite 2-Dodecanone in the Model Hymenopteran Nasonia vitripennis. Toxics 2024, 12, 159. https://doi.org/10.3390/toxics12020159
Planelló R, Aquilino M, Beaugeard L, Llorente L, Herrero Ó, Siaussat D, Lécureuil C. Unveiling Molecular Effects of the Secondary Metabolite 2-Dodecanone in the Model Hymenopteran Nasonia vitripennis. Toxics. 2024; 12(2):159. https://doi.org/10.3390/toxics12020159
Chicago/Turabian StylePlanelló, Rosario, Mónica Aquilino, Laureen Beaugeard, Lola Llorente, Óscar Herrero, David Siaussat, and Charlotte Lécureuil. 2024. "Unveiling Molecular Effects of the Secondary Metabolite 2-Dodecanone in the Model Hymenopteran Nasonia vitripennis" Toxics 12, no. 2: 159. https://doi.org/10.3390/toxics12020159
APA StylePlanelló, R., Aquilino, M., Beaugeard, L., Llorente, L., Herrero, Ó., Siaussat, D., & Lécureuil, C. (2024). Unveiling Molecular Effects of the Secondary Metabolite 2-Dodecanone in the Model Hymenopteran Nasonia vitripennis. Toxics, 12(2), 159. https://doi.org/10.3390/toxics12020159