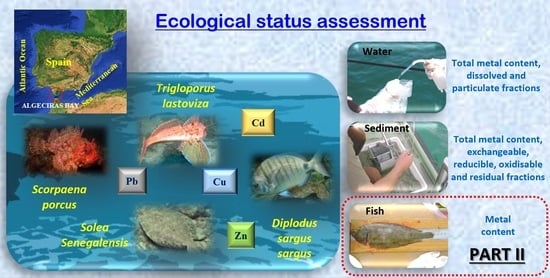
Ecological Status of Algeciras Bay, in a Highly Anthropised Area in South-West Europe, through Metal Assessment—Part II: Biotic Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Area and Sampling Sites
2.2. Equipment and Reagents
2.3. Collection, Pretreatment and Analysis of Biotic Samples
2.4. Quality Control and Quality Assurance
2.5. Statistical Software
3. Results and Discussion
3.1. Metal Content in Fish Samples
3.1.1. Comparison with Guide Levels and Other Ecosystems
3.1.2. Assessment of Fish Quality Using Metal Pollution Index (MPI)
3.2. Bioaccumulation Factors (BAFs)
3.3. Correlation among Fish, Water and Sediment Metal Contents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karayakar, F.; Işık, U.; Cicik, B. Heavy metal levels in economically important fish species sold by fishermen in Karatas (Adana/TURKEY). J. Food Compos. Anal. 2022, 106, 104348. [Google Scholar] [CrossRef]
- Reksten, A.M.; Rahman, Z.; Kjellevold, M.; Gamarro, E.G.; Thilsted, S.H.; Pincus, L.M.; Aakre, I.; Ryder, J.; Ariyawansa, S.; Nordhagen, A.; et al. Metal contents in fish from the Bay of Bengal and potential consumer exposure—The EAF-nansen programme. Foods 2021, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Pupavac, S.M.; Jovanović, G.K.; Linšak, Ž.; Glad, M.; Traven, L.; Žeželj, S.P. The influence on fish and seafood consumption, and the attitudes and reasons for its consumption in the Croatian population. Front. Sustain. Food Syst. 2022, 6, 945186. [Google Scholar] [CrossRef]
- Altiok, S.; Murthy, A.; Iha, K.; Galli, A. Reducing Mediterranean Seafood Footprints: The role of consumer attitudes. Ocean Coast. Manag. 2021, 214, 105915. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Bautista-Covarrubias, J.C.; Osuna-Martínez, C.C.; Delgado-Alvarez, C.; Bojórquez, C.; Aguilar-Juárez, M.; Roos-Muñoz, S.; Osuna-López, I.; Páez-Osuna, F. Metals and Oxidative Stress in Aquatic Decapod Crustaceans: A Review with Special Reference to Shrimp and Crabs. Aquat. Toxicol. 2022, 242, 106024. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ.-Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Jayaprakash, M.; Senthil Kumar, R.; Giridharan, L.; Sujitha, S.B.; Sarkar, S.K.; Jonathan, M.P. Bioaccumulation of Metals in Fish Species from Water and Sediments in Macrotidal Ennore Creek, Chennai, SE Coast of India: A Metropolitan City Effect. Ecotoxicol. Environ. Saf. 2015, 120, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, M.P.; Aurioles-Gamboa, D.; Villegas, L.E.C.; Bohórquez-Herrera, J.; Hernández-Camacho, C.J.; Sujitha, S.B. Metal Concentrations in Demersal Fish Species from Santa Maria Bay, Baja California Sur, Mexico (Pacific Coast). Mar. Pollut. Bull. 2015, 99, 356–361. [Google Scholar] [CrossRef]
- Aytekin, T.; Kargın, D.; Çoğun, H.Y.; Temiz, Ö.; Varkal, H.S.; Kargın, F. Accumulation and Health Risk Assessment of Heavy Metals in Tissues of the Shrimp and Fish Species from the Yumurtalik Coast of Iskenderun Gulf, Turkey. Heliyon 2019, 5, e02131. [Google Scholar] [CrossRef]
- Kazemi, A.; Esmaeilbeigi, M.; Ansari, A.; Asl, A.G.; Mohammadzadeh, B. Alterations and Health Risk Assessment of the Environmental Concentration of Heavy Metals in the Edible Tissue of Marine Fish (Thunnus Tonggol) Consumed by Different Cooking Methods. Reg. Stud. Mar. Sci. 2022, 53, 102361. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Li, X. Bioaccumulation of Heavy Metals in Fish Species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Kalantzi, I.; Mylona, K.; Pergantis, S.A.; Coli, A.; Panopoulos, S.; Tsapakis, M. Elemental Distribution in the Different Tissues of Brood Stock from Greek Hatcheries. Aquaculture 2019, 503, 175–185. [Google Scholar] [CrossRef]
- Vetsis, E.; Kalantzi, I.; Pergantis, S.A.; Kokokiris, L.; Karakassis, I. Metals in Tissues of Marine Fish from the Thermaikos Gulf, Eastern Mediterranean Sea: Detection of Changes with Trophic Level. Mar. Pollut. Bull. 2021, 173, 113024. [Google Scholar] [CrossRef]
- Pan, B.; Wang, Y.; Li, D.; Wang, T.; Du, L. Tissue-Specific Distribution and Bioaccumulation Pattern of Trace Metals in Fish Species from the Heavily Sediment-Laden Yellow River, China. J. Hazard. Mater. 2022, 425, 128050. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Mora-Medina, R.; Fazio, F.; Parrino, V.; Ayala-Soldado, N. Determination of the Heavy Metal Bioaccumulation Patterns in Muscles of Two Species of Mullets from the Southern Caspian Sea. Animals 2022, 12, 2819. [Google Scholar] [CrossRef]
- Monier, M.N.; Soliman, A.M.; Al-Halani, A.A. The Seasonal Assessment of Heavy Metals Pollution in Water, Sediments, and Fish of Grey Mullet, Red Seabream, and Sardine from the Mediterranean Coast, Damietta, North Egypt. Reg. Stud. Mar. Sci. 2023, 57, 102744. [Google Scholar] [CrossRef]
- Allaby, M. A Dictionary of Zoology (Oxford Quick Reference), 5th ed.; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Rahman, M.S.; Akther, S.; Ahmed, A.S.S.; Saha, N.; Rahman, L.S.; Ahmed, M.K.; Arai, T.; Idris, A.M. Distribution and source apportionment of toxic and trace elements in some benthic and pelagic coastal fish species in Karnaphuli River Estuary, Bangladesh: Risk to human health. Mar. Pollut. Bull. 2022, 183, 114044. [Google Scholar] [CrossRef]
- Chan, W.S.; Routh, J.; Luo, C.; Dario, M.; Miao, Y.; Luo, D.; Wei, L. Metal Accumulations in Aquatic Organisms and Health Risks in an Acid Mine-Affected Site in South China. Environ. Geochem. Health 2021, 43, 4415–4440. [Google Scholar] [CrossRef]
- Monikh, F.A.; Safahieh, A.; Savari, A.; Ronagh, M.T.; Doraghi, A. The Relationship between Heavy Metal (Cd, Co, Cu, Ni and Pb) Levels and the Size of Benthic, Benthopelagic and Pelagic Fish Species, Persian Gulf. Bull. Environ. Contam. Toxicol. 2013, 90, 691–696. [Google Scholar] [CrossRef]
- Man, X.; Huang, H.; Chen, F.; Gu, Y.; Liang, R.; Wang, B.; Jordan, R.W.; Jiang, S. Anthropogenic Impacts on the Temporal Variation of Heavy Metals in Daya Bay (South China). Mar. Pollut. Bull. 2022, 185, 114209. [Google Scholar] [CrossRef] [PubMed]
- Anagha, B.; Athira, P.S.; Anisha, P.; Charles, P.E.; Anandkumar, A.; Rajaram, R. Biomonitoring of Heavy Metals Accumulation in Molluscs and Echinoderms Collected from Southern Coastal India. Mar. Pollut. Bull. 2022, 184, 114169. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Sokolova, I.M. Interactive Effects of Metal Pollution and Ocean Acidification on Physiology of Marine Organisms. Curr. Zool. 2015, 61, 653–668. [Google Scholar] [CrossRef]
- Layglon, N.; Abdou, M.; Massa, F.; Castellano, M.; Bakker, E.; Povero, P.; Tercier-Waeber, M.-L. Speciation of Cu, Cd, Pb and Zn in a Contaminated Harbor and Comparison to Environmental Quality Standards. J. Environ. Manag. 2022, 317, 115375. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Gracia, I. Potential Mobility of Metals in Polluted Coastal Sediments in Two Bays of Southern Spain. J. Coast. Res. 2007, 2007, 352–361. [Google Scholar] [CrossRef]
- Sánchez-Garrido, J.C.; Lafuente, J.G.; Sammartino, S.; Naranjo, C.; de los Santos, F.J.; Álvarez Fanjul, E. Meteorologically-Driven Circulation and Flushing Times of the Bay of Algeciras, Strait of Gibraltar. Mar. Pollut. Bull. 2014, 80, 97–106. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística. Cifras Oficiales de Población de los Municipios Españoles en Aplicación de la Ley de Bases del Régimen Local (Art. 17). Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=2864&L=0 (accessed on 25 October 2023).
- Epdata. Gibraltar—Cuántos Habitantes Tiene el País, Datos Demográficos. Available online: https://www.epdata.es/datos/habitantes-pais-poblacion-datos-estadisticas-demograficos/670/gibraltar/119 (accessed on 25 October 2023).
- Araújo, C.V.M.; Diz, F.R.; Tornero, V.; Lubián, L.M.; Blasco, J.; Moreno-Garrido, I. Ranking Sediment Samples from Three Spanish Estuaries in Relation to Its Toxicity for Two Benthic Species: The Microalga Cylindrotheca Closterium and the Copepod Tisbe Battagliai. Environ. Toxicol. Chem. 2010, 29, 393–400. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J. Trace Metal Bioavailability in the Waters of Two Different Habitats in Spain: Huelva Estuary and Algeciras Bay. Ecotoxicol. Environ. Saf. 2008, 71, 851–859. [Google Scholar] [CrossRef]
- Periáñez, R. Modelling the Environmental Behaviour of Pollutants in Algeciras Bay (South Spain). Mar. Pollut. Bull. 2012, 64, 221–232. [Google Scholar] [CrossRef]
- García Sarasa, C. Especies de Interés Pesquero en el Litoral de Andalucía: Vertebrados; Junta de Andalucía, Consejería de Agricultura y Pesca: Sevilla, Spain, 2001; Volume 1, p. 444. Available online: https://www.juntadeandalucia.es/export/drupaljda/Especies_Pesquera_baja.pdf (accessed on 25 October 2023).
- Kosore, C.M.; Galindo-Riaño, M.D.; Díaz-de-Alba, M. Assessing Trace-Element Mobility in Algeciras Bay (Spain) Sediments by Acid and Complexing Screening. Arab. J. Chem. 2015, 12, 2992–3003. [Google Scholar] [CrossRef]
- Vicente-Martorell, J.J.; Galindo-Riaño, M.D.; García-Vargas, M.; Granado-Castro, M.D. Bioavailability of Heavy Metals Monitoring Water, Sediments and Fish Species from a Polluted Estuary. J. Hazard. Mater. 2009, 162, 823–836. [Google Scholar] [CrossRef]
- Arain, M.B.; Kazi, T.G.; Jamali, M.K.; Baig, J.A.; Afridi, H.I.; Jalbani, N.; Sarfraz, R.A. Comparison of Different Extraction Approaches for Heavy Metal Partitioning in Sediment Samples. Pedosphere 2009, 19, 476–485. [Google Scholar] [CrossRef]
- McGeer, J.C.; Brix, K.V.; Skeaff, J.M.; Deforest, D.K.; Brigham, S.I.; Adams, W.J.; Green, A. Inverse Relationship between Bioconcentration Factor and Exposure Concentration for Metals: Implications for Hazard Assessment of Metals in the Aquatic Environment. Environ. Toxicol. Chem. 2003, 22, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Merciai, R.; Guasch, H.; Kumar, A.; Sabater, S.; García-Berthou, E. Trace metal concentration and fish size: Variation among fish species in a Mediterranean river. Ecotoxicol. Environ. Saf. 2014, 107, 154–161. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Varol, M.; Kaçar, E.; Sünbül, M.R.; Towfiqul Islam, A.R.M. Species, Tissue and Gender-Related Metal and Element Accumulation in Fish Species in a Large Reservoir (Turkey) and Health Risks and Nutritional Benefits for Consumers. Environ. Toxicol. Pharmacol. 2022, 94, 103929. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.B.; Aris, A.Z.; Munusamy, V.; Praveena, S.M. Assessment level of heavy metals in Penaeus monodon and Oreochromis spp. in selected aquaculture ponds of high densities development area. Eur. J. Sci. Res. 2009, 30, 348–360. [Google Scholar]
- FAO/WHO. Evaluation of Certain Food Additives and the Contaminants Mercury, Lead and Cadmium; WHO Technical Report Series No. 505; FAO: Rome, Italy; WHO: Geneva, Switzerland, 1989.
- MAFF (Ministry of Agriculture, Fisheries and Food). Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea. In Aquatic Environment Monitoring Report No. 52; The Centre for Environment, Fisheries and Aquaculture Science: Lowestoft, UK, 1998. [Google Scholar]
- EC (European Community). Commission Regulation No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- EU. Commission regulation (EU) No. 488/2014 of 12 May 2014 amending regulation (EC) No. 1881/2006 as regards maximum levels of cadmium in foodstuffs. L 138/75. Off. J. Eur. Union. 2014, 138, 75–79. Available online: https://www.fsai.ie/uploadedFiles/Reg488_2014.pdf (accessed on 25 October 2023).
- EU. Commission regulation (EU) No. 2015/1005 of 25 June 2015 amending regulation (EC) No. 1881/2006 as regards maximum levels of lead in certain foodstuffs. L 161/9. Off. J. Eur. Union. 2015, 161, 9–13. Available online: https://faolex.fao.org/docs/pdf/eur146524.pdf (accessed on 25 October 2023).
- Dural, M.; Lugal Göksu, M.Z.; Özak, A.A.; Derici, B. Bioaccumulation of some heavy metals in different tissues of Dicentrarchus Labrax L, 1758, Sparus Aurata L, 1758 and Mugil cephalus L, 1758 from the ÇamlIk lagoon of the eastern cost of Mediterranean (Turkey). Environ. Monit. Assess. 2006, 118, 65–74. [Google Scholar] [CrossRef]
- Galindo, M.D.; Jurado, J.A.; García, M.; González de Canales, M.L.; Oliva, M.; López, F.; Granado, M.D.; Espada, E. Trace Metal Accumulation in Tissues of Sole (Solea senegalensis) and the Relationships with the Abiotic Environment. Int. J. Environ. Anal. Chem. 2012, 92, 1072–1092. [Google Scholar] [CrossRef]
- Diop, M.; Howsam, M.; Diop, C.; Cazier, F.; Goossens, J.F.; Diouf, A.; Amara, R. Spatial and Seasonal Variations of Trace Elements Concentrations in Liver and Muscle of Round Sardinelle (Sardinella Aurita) and Senegalese Sole (Solea Senegalensis) along the Senegalese Coast. Chemosphere 2016, 144, 758–766. [Google Scholar] [CrossRef]
- Ourgaud, M.; Ruitton, S.; Bourgogne, H.; Bustamante, P.; Churlaud, C.; Guillou, G.; Lebreton, B.; Harmelin-Vivien, M.L. Trace Elements in a Mediterranean Scorpaenid Fish: Bioaccumulation Processes and Spatial Variations. Prog. Oceanogr. 2018, 163, 184–195. [Google Scholar] [CrossRef]
- Bonsignore, M.; Salvagio Manta, D.; Mirto, S.; Quinci, E.M.; Ape, F.; Montalto, V.; Gristina, M.; Traina, A.; Sprovieri, M. Bioaccumulation of Heavy Metals in Fish, Crustaceans, Molluscs and Echinoderms from the Tuscany Coast. Ecotoxicol. Environ. Saf. 2018, 162, 554–562. [Google Scholar] [CrossRef]
- Bat, L.; Öztekin, A.; Şahin, F. Heavy Metal Detection in Scorpaena Porcus Linnaeus, 1758 from Sinop Coast of the Black Sea and Potential Risks to Human Health. Curr. Agric. Res. J. 2018, 6, 255–260. [Google Scholar] [CrossRef]
- Çulha, S.T.; Yabanlı, M.; Baki, B.; Yozukmaz, A. Heavy Metals in Tissues of Scorpionfish (Scorpaena porcus) Caught from Black Sea (Turkey) and Potential Risks to Human Health. Environ. Sci. Pollut. Res. 2016, 23, 20882–20892. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Chekri, R.; Leufroy, A.; Jitaru, P.; Millour, S.; Marchond, N.; Chafey, C.; Testu, C.; Zinck, J.; Cresson, P.; et al. Trace Element Contamination in Fish Impacted by Bauxite Red Mud Disposal in the Cassidaigne Canyon (NW French Mediterranean). Sci. Total Environ. 2019, 690, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Uluozlu, O.D.; Tuzen, M.; Mendil, D.; Soylak, M. Trace Metal Content in Nine Species of Fish from the Black and Aegean Seas, Turkey. Food Chem. 2007, 104, 835–840. [Google Scholar] [CrossRef]
- Scopelliti, G.; Di Leonardo, R.; Tramati, C.D.; Mazzola, A.; Vizzini, S. Premature Aging in Bone of Fish from a Highly Polluted Marine Area. Mar. Pollut. Bull. 2015, 97, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.; Gutiérrez, Á.J.; Lozano, G.; González-Weller, D.; Lozano-Bilbao, E.; Rubio, C.; Caballero, J.M.; Revert, C.; Hardisson, A. Metals in Diplodus sargus cadenati and Sparisoma cretense—A Risk Assessment for Consumers. Environ. Sci. Pollut. Res. 2018, 25, 2630–2642. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Caetano, M.; Costa, J.; Pousão-Ferreira, P.; Vale, C.; Reis-Henriques, M.A. Metal Accumulation and Oxidative Stress Responses in, Cultured and Wild, White Seabream from Northwest Atlantic. Sci. Total Environ. 2008, 407, 638–646. [Google Scholar] [CrossRef]
- Caçador, I.; Costa, J.L.; Duarte, B.; Silva, G.; Medeiros, J.P.; Azeda, C.; Castro, N.; Freitas, J.; Pedro, S.; Almeida, P.R.; et al. Macroinvertebrates and Fishes as Biomonitors of Heavy Metal Concentration in the Seixal Bay (Tagus Estuary): Which Species Perform Better? Ecol. Indic. 2012, 19, 184–190. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Brach-Papa, C.; Gonzalez, J.L.; Lenfant, P.; Darnaude, A.M. Growth, Condition and Metal Concentration in Juveniles of Two Diplodus Species in Ports. Mar. Pollut. Bull. 2018, 126, 31–42. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Baeza-Rojano, E.; Cabezas, M.P.; Díaz-Pavón, J.J.; Pacios, I.; García-Gómez, J.C. The Amphipods Caprella penantis and Hyale schmidtii as Biomonitors of Trace Metal Contamination in Intertidal Ecosystems of Algeciras Bay, Southern Spain. Mar. Pollut. Bull. 2009, 58, 783–786. [Google Scholar] [CrossRef]
- Usero, J.; González-Regalado, E.; Gracia, I. Trace Metals in the Bivalve Molluscs Ruditapes Decussatus and Ruditapes Philippinarum from the Atlantic Coast of Southern Spain. Environ. Int. 1997, 23, 291–298. [Google Scholar] [CrossRef]
- Usero, J.; Izquierdo, C.; Morillo, J.; Gracia, I. Heavy metals in fish (Solea vulgaris, Anguilla anguilla and Liza aurata) from salt marshes on the southern Atlantic coast of Spain. Environ. Int. 2003, 29, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Töre, Y.; Ustaoğlu, F.; Tepe, Y.; Kalipci, E. Levels of Toxic Metals in Edible Fish Species of the Tigris River (Turkey); Threat to Public Health. Ecol. Indic. 2021, 123, 107361. [Google Scholar] [CrossRef]
- Zaghloul, G.Y.; Ezz El-Din, H.M.; Mohamedein, L.I.; El-Moselhy, K.M. Bio-Accumulation and Health Risk Assessment of Heavy Metals in Different Edible Fish Species from Hurghada City, Red Sea, Egypt. Environ. Toxicol. Pharmacol. 2022, 95, 103969. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, W.; Li, F.; Pan, Y.; Wang, C.; Tian, H. Occurrence, partition, and risk of seven heavy metals in sediments, seawater, and organisms from the eastern sea area of Shandong Peninsula, Yellow Sea, China. J. Environ. Manag. 2021, 279, 111771. [Google Scholar] [CrossRef]
- Kontas, A.; Uluturhan, E.; Alyuruk, H.; Darilmaz, E.; Bilgin, M.; Altay, O. Metal Contamination in Surficial Sediments of Edremit Bay (Aegean Sea): Spatial Distribution, Source Identification and Ecological Risk Assessment. Reg. Stud. Mar. Sci. 2020, 40, 101487. [Google Scholar] [CrossRef]
- Morais, S.; Aragão, C.; Cabrita, E.; Conceição, L.E.C.; Constenla, M.; Costas, B.; Dias, J.; Duncan, N.; Engrola, S.; Estevez, A.; et al. New Developments and Biological Insights into the Farming of Solea Senegalensis Reinforcing Its Aquaculture Potential. Rev. Aquac. 2016, 8, 227–263. [Google Scholar] [CrossRef]
- Ghribi, R.; Correia, A.T.; Elleuch, B.; Nunes, B. Testing the Impact of Contaminated Sediments from the Southeast Marine Coast of Tunisia on Biota: A Multibiomarker Approach Using the Flatfish Solea Senegalensis. Environ. Sci. Pollut. Res. 2019, 26, 29704–29721. [Google Scholar] [CrossRef]
- Riba, I.; Casado-Martínez, M.C.; Blasco, J.; DelValls, T.A. Bioavailability of Heavy Metals Bound to Sediments Affected by a Mining Spill Using Solea Senegalensis and Scrobicularia Plana. Mar. Environ. Res. 2004, 58, 395–399. [Google Scholar] [CrossRef]
- Salamanca, M.J.; Jiménez-Tenorio, N.; Gonzalez de Canales, M.L.; DelValls, T.A. Determinación de La Toxicidad de Un Vertido de Petróleo Mediante El Uso de Bioensayos Con El Pez Solea Senegalensis. Cienc. Mar. 2008, 34, 339–348. [Google Scholar] [CrossRef]
- Solé, M.; Lima, D.; Reis-Henriques, M.A.; Santos, M.M. Stress Biomarkers in Juvenile Senegal Sole, Solea Senegalensis, Exposed to the Water-Accommodated Fraction of the “Prestige” Fuel Oil. Bull. Environ. Contam. Toxicol. 2008, 80, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Vega, S.; Varó, I. Characterization of Type “B” Esterases and Hepatic CYP450 Isoenzimes in Senegalese Sole for Their Further Application in Monitoring Studies. Ecotoxicol. Environ. Saf. 2012, 78, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Tenorio, N.; Salamanca, M.J.; García-Luque, E.; Gonzalez de Canales, M.L.; DelValls, T.A. Chronic Bioassay in Benthic Fish for the Assessment of the Quality of Sediments in Different Areas of the Coast of Spain Impacted by Acute and Chronic Oil Spills. Environ. Toxicol. 2008, 23, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Diniz, M.S.; Caeiro, S.; Lobo, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; DelValls, T.Á.; Costa, M.H. Histological Biomarkers in Liver and Gills of Juvenile Solea Senegalensis Exposed to Contaminated Estuarine Sediments: A Weighted Indices Approach. Aquat. Toxicol. 2009, 92, 202–212. [Google Scholar] [CrossRef]
- Costa, P.M.; Caeiro, S.; Lobo, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; DelValls, T.Á.; Costa, M.H. Estuarine Ecological Risk Based on Hepatic Histopathological Indices from Laboratory and in Situ Tested Fish. Mar. Pollut. Bull. 2011, 62, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; José Vicente, J.; Gravato, C.; Guilhermino, L.; Dolores Galindo-Riaño, M. Oxidative Stress Biomarkers in Senegal Sole, Solea Senegalensis, to Assess the Impact of Heavy Metal Pollution in a Huelva Estuary (SW Spain): Seasonal and Spatial Variation. Ecotoxicol. Environ. Saf. 2012, 75, 151–162. [Google Scholar] [CrossRef]
- Oliva, M.; Perales, J.A.; Gravato, C.; Guilhermino, L.; Galindo-Riaño, M.D. Biomarkers Responses in Muscle of Senegal Sole (Solea Senegalensis) from a Heavy Metals and PAHs Polluted Estuary. Mar. Pollut. Bull. 2012, 64, 2097–2108. [Google Scholar] [CrossRef]
- Fonseca, V.F.; Vasconcelos, R.P.; Tanner, S.E.; França, S.; Serafim, A.; Lopes, B.; Company, R.; Bebianno, M.J.; Costa, M.J.; Cabral, H.N. Habitat Quality of Estuarine Nursery Grounds: Integrating Non-Biological Indicators and Multilevel Biological Responses in Solea Senegalensis. Ecol. Indic. 2015, 58, 335–345. [Google Scholar] [CrossRef]
- Martins, C.; Alves de Matos, A.P.; Costa, M.H.; Costa, P.M. Alterations in Juvenile Flatfish Gill Epithelia Induced by Sediment-Bound Toxicants: A Comparative in Situ and Ex Situ Study. Mar. Environ. Res. 2015, 112, 122–130. [Google Scholar] [CrossRef]
- Briaudeau, T.; Zorita, I.; Cuevas, N.; Franco, J.; Marigómez, I.; Izagirre, U. Multi-Annual Survey of Health Status Disturbance in the Bilbao Estuary (Bay of Biscay) Based on Sediment Chemistry and Juvenile Sole (Solea spp.) Histopathology. Mar. Pollut. Bull. 2019, 145, 126–137. [Google Scholar] [CrossRef]
- La Colla, N.S.; Botté, S.E.; Simonetti, P.; Negrin, V.L.; Serra, A.V.; Marcovecchio, J.E. Water, Sediments and Fishes: First Multi Compartment Assessment of Metal Pollution in a Coastal Environment from the SW Atlantic. Chemosphere 2021, 282, 131131. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Masunaga, S. Assessment of Trace Metals in Fish Species of Urban Rivers in Bangladesh and Health Implications. Environ. Toxicol. Pharmacol. 2015, 39, 347–357. [Google Scholar] [CrossRef]
- Avigliano, E.; Monferrán, M.V.; Sánchez, S.; Wunderlin, D.A.; Gastaminza, J.; Volpedo, A.V. Distribution and Bioaccumulation of 12 Trace Elements in Water, Sediment and Tissues of the Main Fishery from Different Environments of the La Plata Basin (South America): Risk Assessment for Human Consumption. Chemosphere 2019, 236, 124394. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.S.; Rahman, M.; Sultana, S.; Babu, S.M.O.F.; Sarker, M.S.I. Bioaccumulation and Heavy Metal Concentration in Tissues of Some Commercial Fishes from the Meghna River Estuary in Bangladesh and Human Health Implications. Mar. Pollut. Bull. 2019, 145, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Rubalingeswari, N.; Thulasimala, D.; Giridharan, L.; Gopal, V.; Magesh, N.S.; Jayaprakash, M. Bioaccumulation of Heavy Metals in Water, Sediment, and Tissues of Major Fisheries from Adyar Estuary, Southeast Coast of India: An Ecotoxicological Impact of a Metropolitan City. Mar. Pollut. Bull. 2021, 163, 111964. [Google Scholar] [CrossRef] [PubMed]
- Adani, P.; Sawale, A.A.; Nandhagopal, G. Bioaccumulation of Heavy Metals in the Food Components from Water and Sediments in the Coastal Waters of Kalpakkam, Southeast Coast of India. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100627. [Google Scholar] [CrossRef]
- Beg, M.U.; Al-Jandal, N.; Al-Subiai, S.; Karam, Q.; Husain, S.; Butt, S.A.; Ali, A.; Al-Hasan, E.; Al-Dufaileej, S.; Al-Husaini, M. Metallothionein, Oxidative Stress and Trace Metals in Gills and Liver of Demersal and Pelagic Fish Species from Kuwaits’ Marine Area. Mar. Pollut. Bull. 2015, 100, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in Aquatic Invertebrates: Their Role in Metal Detoxification and Their Use as Biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological Risk Assessment of Heavy Metals in Sediment and Human Health Risk Assessment of Heavy Metals in Fishes in the Middle and Lower Reaches of the Yangtze River Basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef]



| Site | Tissue | Zn | Cd | Pb | Cu |
|---|---|---|---|---|---|
| 1 Getares beach | Gills (n = 40) | (6–1153) 98 ± 196 | (<LD) <LD | (<LD–39.1) 3.05 ± 7.35 | (0.6–10.8) 3.22 ± 1.60 |
| Liver (n = 33) | (17–503) 121 ± 86 | (0.06–2.90) 0.96 ± 0.70 | (<LD–7.71) 2.76 ± 2.05 | (4–1492) 313 ± 335 | |
| Muscle (n = 40) | (12.3–43.8) 20.1 ± 7.5 | (<LD) <LD | (<LD–3.13) <LD a | (0.28–1.98) 0.82 ± 0.37 | |
| 2 Isla Verde | Gills (n = 9) | (38.5–80.7) 61.2 ± 12.0 | (<LD–0.21) <LD | (<LD–4.63) 1.25 ± 1.45 | (1.20–3.78) 2.45 ± 0.93 b |
| Liver (n = 7) | (72–475) 207 ± 137 | (0.23–3.83) 1.36 ± 1.55 | (<LD–2.06) 0.73 ± 0.81 | (9–636) 104 ± 234 | |
| Muscle (n = 8) | (12.5–46.6) 24.0 ± 11.5 | (<LD) <LD | (<LD) <LD | (0.30–2.02) 0.74 ± 0.67 | |
| 3 Palmones | Gills (n = 18) | (<LD–74.30) 65.8 ± 17.4 | (<LD–0.10) <LD | (<LD–7.06) 2.14 ± 1.78 | (<LD–31.83) 4.37 ± 8.23 |
| Liver (n = 18) | (68–250) 127 ± 44 | (<LD–2.53) 0.38 ± 0.58 | (<LD–5.12) 1.03 ± 1.39 | (10–426) 92 ± 132 | |
| Muscle (n = 21) | (<LD–260) 30.0 ± 53.1 | (<LD–0.40) <LD | (<LD–1.06) <LD | (<LD–2.48) 1.17 ± 0.64 c | |
| 4 Guadarranque | Gills (n = 12) | (58–111) 69.7 ± 19.5 | (<LD–0.11) <LD | (<LD–2.31) 0.76 ± 0.74 | (1.39–9.87) 2.91 ± 2.28 |
| Liver (n = 11) | (14–299) 139 ± 90 | (<LD–1.73) 0.79 ± 0.51 | (<LD–2.56) 0.88 ± 0.96 | (7–262) 69.1 ± 89.6 | |
| Muscle (n = 11) | (11.7–41.5) 22.0 ± 8.0 | (<LD–0.10) <LD | (<LD–1.49) 0.20 ± 0.45 | (0.4–10.1) 1.55 ± 2.86 | |
| 5 Puente Mayorga | Gills (n = 8) | (<LD–151) 67.7 ± 41.4 | (<LD–0.52) <LD | (0.51–8.20) 2.58 ± 3.06 | (2.1–34.1) 7.4 ± 10.8 |
| Liver (n = 7) | (63–127) 104 ± 27 | (<LD–1.22) 0.47 ± 0.48 | (<LD–7.56) 2.35 ± 2.90 | (2–1505) 268 ± 548 | |
| Muscle (n = 7) | (16.3–25.5) 19.5 ± 3.0 | (<LD) <LD | (<LD–0.29) <LD | (0.57–2.10) 1.03 ± 0.53 |
| Organisation a | Zn | Cd | Pb | Cu | Reference |
|---|---|---|---|---|---|
| FAO maximum limit for fish | 30–100 (143–476) b | 0.05–5.5 (0.24–26.2) b | 0.5–6.0 (0.11–28.6) b | 10–100 (47.6–476) b | [41] |
| WHO 1989 | 100 (476) b | 1 (4.8) b | 2 (9.6) b | 30 (143) b | [42] |
| England MAFF | 50 (238) b | 0.2 (0.96) b | 2 (9.6) b | 20 (95.2) b | [43] |
| EC, EU | 0.05 (0.24) b | 0.3 (1.4) b | [44,45,46] | ||
| TFC | 50 (238) b | 0.1 (0.48) b | 1 (4.8) b | 20 (95.2) b | [47] |
| Algeciras Bay | Zn | Cd | Pb | Cu | Reference |
| Minimum-maximum values | This study | ||||
| sole | <LD–32.3 | <LD–0.1 | <LD–0.35 | <LD–10.1 | |
| black scorpionfish | 14.0–41.5 | <LD | <LD–3.1 | 0.3–2.0 | |
| streaked gurnard | 12–260 | <LD–0.4 | <LD–1.5 | 0.4–2.5 | |
| white seabream | 16.0–46.6 | <LD | <LD–0.9 | 0.3–1.3 | |
| Percentage surpassing the most restrictive FAO limits c | |||||
| sole | 0% | 0% | 13% | 0% | |
| black scorpionfish | 0% | 0% | 27% | 0% | |
| streaked gurnard | 5% | 5% | 19% | 0% | |
| white seabream | 0% | 0% | 20% | 0% | |
| Tissue | Zn | Cd | Pb | Cu | Site | Reference |
|---|---|---|---|---|---|---|
| Sole | ||||||
| Gills | 58.5 | <LD | 2.76 | 5.29 | Algeciras Bay (Spain) | This study |
| Liver | 98.7 | 0.84 | 2.95 | 356 | ||
| Muscle | 17.5 | <LD | <LD | 0.98 | ||
| Gills | 73.7 (−1.3) | 0.56 | 4.52 (−1.6) | 11.9 (−2.2) | Huelva Estuary (Spain) | [35] |
| Liver | 88.2 (+1.1) | 1.82 (−2.2) | 1.46 (+2.0) | 433.5 (−1.2) | ||
| Muscle | 23.7 (−1.4) | 0.01 | 0.4 | 1.41 (−1.4) | ||
| Gills | 94.5 (−1.6) | 0.4 | 2.72 (1.0) | 5.12 (1.0) | Cádiz Bay (Spain) | [48] |
| Liver | 120.8 (−1.2) | 0.55 (+1.5) | 0.086 (+34.3) | 441.6 (−1.2) | ||
| Muscle | 26.1 (−1.5) | 0.1 | 0.01 | 0.78 (+1.3) | ||
| Liver | 78.0 (+1.3) | 0.7 (+1.2) | 0.14 (+20.9) | 426.5 (−1.2) | Senegalese coasts (Africa) | [49] |
| Muscle | 17.4 (1.0) | <LD | <LD | 0.49 (+2.0) | ||
| Black scorpionfish | ||||||
| Gills | 68.2 | 0.014 | 0.76 | 2.28 | Algeciras Bay (Spain) | This study |
| Liver | 223.7 | 1.01 | 0.20 | 17.6 | ||
| Muscle | 26.3 | <LD | 0.32 | 0.98 | ||
| Muscle | 43.2 (−1.6) | 0.024 | 0.026 (+12.1) | 0.56 (+1.7) | Northwestern Mediterranean Sea (France) | [50] |
| Muscle | 4.37 (+6.0) | 0.002 | 0.013 (+24.9) | 0.22 (+4.5) | Tuscany coast (Italy) | [51] |
| Muscle | 2.40 (+11.0) | 0.01 | 0.02 (+15.9) | 0.10 (+9.8) | Black Sea (Turkey) | [52] |
| Gills | <LD | 0.04 (+19.1) | 0.06 (+38.0) | Black Sea (Turkey) | [53] | |
| Liver | <LD | 0.03 (+6.7) | 0.45 (+39.2) | |||
| Muscle | 0.02 | 0.04 (+7.9) | 0.07 (+14.0) | |||
| Muscle | 0.001 | 0.04 (+7.8) | Cassidaigne Canyon (France) | [54] | ||
| Muscle | 95.3 (−3.6) | 0.80 | 0.66 (−2.1) | 0.73 (+1.3) | Black and Aegean Seas (Turkey) | [55] |
| Muscle | 10 (+2.6) | 0.5 (−1.6) | Augusta Bay (Italy) | [56] | ||
| White seabream | ||||||
| Gills | 198.5 | <LD | 6.87 | 2.23 | Algeciras Bay (Spain) | This study |
| Liver | 113.2 | 1.50 | 2.09 | 133.9 | ||
| Muscle | 32.5 | <LD | 0.19 | 0.88 | ||
| Liver | 83.0 (+1.4) | 2.67 (−1.8) | 0.21 (+10.0) | 23.7 (+5.7) | Gran Canaria (Canary Islands, Spain) | [57] |
| Muscle | 4.51 (+7.2) | 0.003 | 0.02 (+11.0) | 0.57 (+1.5) | ||
| Liver | 3.15 (−2.1) | 0.68 (+3.1) | 44.4 (+3.0) | Ria Formosa (Portugal) | [58] | |
| Muscle | 0.005 | 0.036 (+5.2) | 1.52 (−1.7) | |||
| Muscle | 17.3 (+1.9) | 1.48 | 10.6 (−56.7) | 2.91 (−3.3) | Seixal Bay (Portugal) | [59] |
| Muscle | 28.8 (+1.1) | 0.11 (+1.8) | 2.25 (−2.6) | Bay of Toulon (France) | [60] | |
| Muscle | 0.001 | 0.014 (+13.4) | Cassidaigne Canyon (France) | [54] | ||
| Muscle | 46.1 (−1.4) | 0.11 | 1.99 (−10.6) | 1.53 (−1.7) | Cádiz Bay (Spain) | [61] |
| Fish Specie | MPI | Site | Reference | |||
|---|---|---|---|---|---|---|
| Gills | Liver | Muscle | ||||
| S. senegalensis (sole) | 2.81 | 15.8 | 1.34 | Algeciras Bay (Spain) | This study | |
| S. porcus (black scorpionfish) | 1.80 | 5.21 | 2.18 | |||
| T. lastoviza (streaked gurnard) | 2.15 | 4.75 | 0.73 | |||
| D. sargus sargus (white seabream) | 3.71 | 12.5 | 3.41 | |||
| S. senegalensis | 4.79 | 7.09 | 0.38 | Cádiz Bay (Spain) | [48] | |
| - | 7.55 | 0.27 | Senegalese coast (Africa) | [49] | ||
| S. porcus | - | - | 0.35 | Northwestern Mediterranean Sea (France) | [50] | |
| - | - | 0.07 | Tuscany coast (Italy) | [51] | ||
| - | - | 0.08 | Black Sea area | [52] | ||
| - | - | 2.46 | Black and Aegean Seas (Turkey) | [55] | ||
| D. sargus sargus | - | - | 5.30 | Seixal Bay (Portugal) | [59] | |
| - | - | 1.98 | Cadiz Bay (Spain) | [61] | ||
| D. sargus cadenati | - | 5.76 | 0.10 | North coast of Gran Canaria (Canary Islands) | [57] | |
| D. vulgaris | - | - | 0.87 | Cádiz Bay (Spain) | [61] | |
| M. barbatus | - | - | 1.42 | |||
| M. surmuletus | - | - | 0.98 | |||
| P. acarne | - | - | 1.54 | |||
| P. erythrinus | - | - | 1.09 | |||
| P. auriga | - | - | 1.49 | |||
| P. pagrus | - | - | 1.27 | |||
| S. aurata | - | - | 1.17 | |||
| L. aurata | - | 6.37 | 0.23 | Odiel Estuary (Spain) | [63] | |
| A. anguilla | - | 3.95 | 0.42 | |||
| S. vulgaris | - | 4.15 | 0.27 | |||
| L. aurata | - | 2.13 | 0.16 | Cadiz Bay (Spain) | ||
| A. anguilla | - | 2.39 | 0.26 | |||
| S. vulgaris | - | 2.02 | 0.18 | |||
| S. senegalensis | 6.86 | 17.85 | 0.60 | Ría de Huelva (Spain) | [35] | |
| S. aurata | 4.35 | 27.93 | 0.72 | |||
| L. abu | 1.02 | - | 0.75 | Tigris river (Turkey) | [64] | |
| C. regium | 0.62 | - | 0.42 | |||
| C. macrostomus | 0.94 | - | 0.64 | |||
| B. mystaceus | 0.19 | - | 0.19 | |||
| C. trutta | 0.13 | - | 0.16 | |||
| C. gibelio | 0.16 | - | 0.36 | |||
| G. oyena | 2.19 | 2.83 | 0.54 | Hurghada City, Red Sea (Egypt) | [65] | |
| S. sordidus | 1.45 | 0.75 | 0.62 | |||
| L. lentjan | 1.62 | 3.63 | 0.81 | |||
| S. rivulatus | 1.60 | 13.61 | 0.43 | |||
| M. vanicolensis | 2.81 | 3.35 | 0.98 | |||
| S. solea | 32.29 | 80.87 | 11.53 | Iskenderun Gulf (Turkey) | [9] | |
| S. aurata | 22.81 | 41.49 | 9.42 | |||
| L. tanakae | - | - | 0.68 | Shandong Peninsula, Yellow Sea (China) | [66] | |
| O. kenojei | - | - | 0.88 | |||
| C. stigmatias | - | - | 0.56 | |||
| C. joyneri | - | - | 0.57 | |||
| S. schlegelii | - | - | 0.63 | |||
| L. litulon | - | - | 0.53 | |||
| P. polyactis | - | - | 0.92 | |||
| P. indicus | - | - | 0.68 | |||
| L. micropterus | - | - | 0.40 | |||
| S. niphonius | - | - | 0.69 | |||
| K. punctatus | - | - | 0.56 | |||
| M. cephalus | - | 2.97 | 1.89 | Damietta Port (Egypt) | [17] | |
| P. pagrus | - | 0.97 | 0.56 | |||
| S. aurita | - | 0.65 | 0.33 | |||
| M. merluccius | 1.33 | 5.98 | 0.36 | Edremit Bay, Aegean Sea (Turkey) | [67] | |
| M. barbatus | 0.99 | 4.01 | 0.33 | |||
| P. erythrinus | 1.82 | 11.25 | 0.45 | |||
| Tissue | Factor | Values | Zn | Cd | Pb | Cu |
|---|---|---|---|---|---|---|
| Sole | ||||||
| Gills | BWAF | >1 | 46/46 (100%) | 2/4 (50%) | 27/43 (63%) | 12/13 (92%) |
| >100 | 1/46 (2%) | 0/4 (0%) | 0/43 (0%) | 0/13 (0%) | ||
| BSAF | <1 | 5/46 (11%) | 22/22 (100%) | 43/46 (94%) | 42/46 (92%) | |
| 1–2 | 38/46 (83%) | 0/22 (0%) | 2/46 (4%) | 2/46 (4%) | ||
| >2 | 3/46 (6%) | 0/22 (0%) | 1/46 (2%) | 2/46 (4%) | ||
| Liver | BWAF | >1 | 39/39 (100%) | 2/2 (100%) | 34/36 (94%) | 11/11 (100%) |
| >100 | 8/39 (21%) | 0/2 (0%) | 0/36 (0%) | 6/11 (55%) | ||
| BSAF | <1 | 3/39 (8%) | 6/21 (29%) | 39/39 (100%) | 0/39 (0%) | |
| 1–2 | 9/39 (23%) | 3/21 (14%) | 0/39 (0%) | 3/39 (8%) | ||
| >2 | 27/39 (69%) | 12/21 (57%) | 0/39 (0%) | 36/39 (92%) | ||
| Muscle | BWAF | >1 | 45/46 (98%) | 0/4 (0%) | 0/42 (0%) | 5/12 (42%) |
| >100 | 0/46 (0%) | 0/4 (0%) | 0/42 (0%) | 0/12 (0%) | ||
| BSAF | <1 | 46/46 (100%) | 22/22 (100%) | 45/45 (100%) | 46/46 (100%) | |
| 1–2 | 0/46 (0%) | 0/22 (0%) | 0/45 (0%) | 0/46 (0%) | ||
| >2 | 0/46 (0%) | 0/22 (0%) | 0/45 (0%) | 0/46 (0%) | ||
| Black scorpionfish | ||||||
| Gills | BWAF | >1 | 15/15 (100%) | - | 8/13 (62%) | 1/1 (100%) |
| >100 | 2/15 (13%) | - | 0/13 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 2/15 (13%) | 15/15 (100%) | 15/15 (100%) | 14/14 (100%) | |
| 1–2 | 12/15 (80%) | 0/15 (0%) | 0/15 (0%) | 0/14 (0%) | ||
| >2 | 1/15 (7%) | 0/15 (0%) | 0/15 (0%) | 0/14 (0%) | ||
| Liver | BWAF | >1 | 12/12 (100%) | - | 2/10 (20%) | 1/1 (100%) |
| >100 | 10/12 (83%) | - | 0/10 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 0/12 (0%) | 2/12 (17%) | 12/12 (100%) | 8/12 (67%) | |
| 1–2 | 1/12 (8%) | 1/12 (8%) | 0/12 (0%) | 3/12 (25%) | ||
| >2 | 11/12 (92%) | 9/12 (75%) | 0/12 (0%) | 1/12 (8%) | ||
| Muscle | BWAF | >1 | 15/15 (100%) | - | 3/13 (23%) | 0/1 (0%) |
| >100 | 0/15 (0%) | - | 0/13 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 15/15 (100%) | 15/15 (100%) | 15/15 (100%) | 15/15 (100%) | |
| 1–2 | 0/15 (0%) | 0/15 (0%) | 0/15 (0%) | 0/15 (0%) | ||
| >2 | 0/15 (0%) | 0/15 (0%) | 0/15 (0%) | 0/15 (0%) | ||
| Streaked gurnard | ||||||
| Gills | BWAF | >1 | 19/21 (91%) | 0/12 (0%) | 16/18 (89%) | 1/1 (100%) |
| >100 | 1/21 (5%) | 0/12 (0%) | 0/18 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 14/21 (67%) | 20/20 (100%) | 21/21 (100%) | 21/21 (100%) | |
| 1–2 | 6/21 (28%) | 0/20 (0%) | 0/21 (0%) | 0/21 (0%) | ||
| >2 | 1/21 (5%) | 0/20 (0%) | 0/21 (0%) | 0/21 (0%) | ||
| Liver | BWAF | >1 | 20/20 (100%) | 9/12 (75%) | 9/17 (53%) | 2/2 (100%) |
| >100 | 3/20 (15%) | 0/12 (0%) | 0/17 (0%) | 0/2 (0%) | ||
| BSAF | <1 | 1/20 (5%) | 6/19 (31.5%) | 20/20 (100%) | 12/20 (60%) | |
| 1–2 | 13/20 (65%) | 7/19 (37%) | 0/20 (0%) | 4/20 (20%) | ||
| >2 | 6/20 (30%) | 6/19 (31.5%) | 0/20 (0%) | 4/20 (20%) | ||
| Muscle | BWAF | >1 | 21/21 (100%) | 1/14 (7%) | 2/18 (11%) | 1/1 (100%) |
| >100 | 0/21 (0%) | 0/14 (0%) | 0/18 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 20/21 (95%) | 19/20 (95%) | 21/21 (100%) | 20/20 (100%) | |
| 1–2 | 0/21 (0%) | 0/20 (0%) | 0/21 (0%) | 0/20 (0%) | ||
| >2 | 1/21 (5%) | 1/20 (5%) | 0/21 (0%) | 0/20 (0%) | ||
| White seabream | ||||||
| Gills | BWAF | >1 | 5/5 (100%) | - | 3/3 (100%) | 1/1 (100%) |
| >100 | 1/5 (20%) | - | 0/3 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 0/5 (0%) | 3/3 (100%) | 5/5 (100%) | 5/5 (100%) | |
| 1–2 | 1/5 (20%) | 0/3 (0%) | 0/5 (0%) | 0/5 (0%) | ||
| >2 | 4/5 (80%) | 0/3 (0%) | 0/5 (0%) | 0/5 (0%) | ||
| Liver | BWAF | >1 | 5/5 (100%) | - | 2/3 (67%) | 1/1 (100%) |
| >100 | 2/5 (40%) | - | 0/3 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 0/5 (0%) | 0/3 (0%) | 5/5 (100%) | 1/5 (20%) | |
| 1–2 | 0/5 (0%) | 1/3 (33%) | 0/5 (0%) | 2/5 (40%) | ||
| >2 | 5/5 (100%) | 2/3 (67%) | 0/5 (0%) | 2/5 (40%) | ||
| Muscle | BWAF | >1 | 5/5 (100%) | - | 1/3 (33%) | 0/1 (0%) |
| >100 | 0/5 (0%) | - | 0/3 (0%) | 0/1 (0%) | ||
| BSAF | <1 | 3/5 (60%) | 3/3 (100%) | 5/5 (100%) | 5/5 (100%) | |
| 1–2 | 2/5 (40%) | 0/3 (0%) | 0/5 (0%) | 0/5 (0%) | ||
| >2 | 0/5 (0%) | 0/3 (0%) | 0/5 (0%) | 0/5 (0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanueva-Marenco, M.J.; Galindo-Riaño, M.D.; Granado-Castro, M.D.; Díaz-de-Alba, M. Ecological Status of Algeciras Bay, in a Highly Anthropised Area in South-West Europe, through Metal Assessment—Part II: Biotic Samples. Toxics 2024, 12, 166. https://doi.org/10.3390/toxics12030166
Casanueva-Marenco MJ, Galindo-Riaño MD, Granado-Castro MD, Díaz-de-Alba M. Ecological Status of Algeciras Bay, in a Highly Anthropised Area in South-West Europe, through Metal Assessment—Part II: Biotic Samples. Toxics. 2024; 12(3):166. https://doi.org/10.3390/toxics12030166
Chicago/Turabian StyleCasanueva-Marenco, María José, María Dolores Galindo-Riaño, María Dolores Granado-Castro, and Margarita Díaz-de-Alba. 2024. "Ecological Status of Algeciras Bay, in a Highly Anthropised Area in South-West Europe, through Metal Assessment—Part II: Biotic Samples" Toxics 12, no. 3: 166. https://doi.org/10.3390/toxics12030166
APA StyleCasanueva-Marenco, M. J., Galindo-Riaño, M. D., Granado-Castro, M. D., & Díaz-de-Alba, M. (2024). Ecological Status of Algeciras Bay, in a Highly Anthropised Area in South-West Europe, through Metal Assessment—Part II: Biotic Samples. Toxics, 12(3), 166. https://doi.org/10.3390/toxics12030166