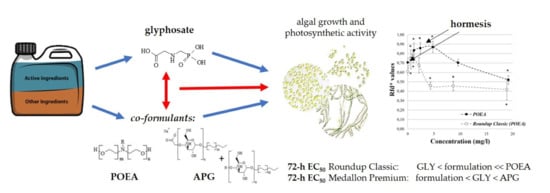
Hormesis, the Individual and Combined Phytotoxicity of the Components of Glyphosate-Based Formulations on Algal Growth and Photosynthetic Activity
Abstract
:1. Introduction
| Algae Species | Tested Substances | Test Concentrations | Test Period | Tested Parameters | Main Results | Reference |
|---|---|---|---|---|---|---|
| P. subcapitata | technical-grade glyphosate (GLY) acid, GLY-IPA a, Roundup, POEA b | dilution series | 96 h c | growth inhibition | 96 h IC50 d = 3.92 mg a.e. e /L (POEA), 5.81 mg a.e./L (Roundup), 24.7 mg a.e./L (GLY acid), 41.0 mg a.e./L (GLY-IPA) | [15] |
| P. subcapitata | Roundup | 4.7–60 mg/L | 96 h | growth inhibition | 96 h EC50 f = 15.60 mg/L, damaged cell ultrastructure | [52] |
| C. vulgaris | GLY, AMPA g | 0.05–50 mg/L, individual and co-exposures | 7 d h | growth inhibition, pigment content, antioxidant activity | stimulated growth (≤ 0.5 mg/L), growth inhibition (≥ 5 mg/L), inhibitory effect (≥ 5 mg/L GLY and AMPA), altered pigment levels, increased antioxidant activity | [64] |
| cyanobacteria, Chlorophycean microalgae | GBH i (Faena) | 1–100 mg/L | 96 h | growth inhibition, antioxidant enzymes | IC50 = 1.022–2.702 mg/L, affected antioxidant enzyme activity (≥ 0.74 mg/L) | [65] |
| M. aeruginosa | GLY | 1–10 mg/L | 9 d, enzyme assays: 24–48 h | growth inhibition, chl-a j content, antioxidant activity, cell apoptosis | reduced growth and chl-a content, increased antioxidant activity (1–2 mg/L), induced apoptosis | [66] |
| cyanobacterial strains | GLY | 8.5–33.8 mg/L | 15 d | growth inhibition, phosphate and phosphonate levels | species- and dose-dependent stimulatory effects, decreased phosphonate levels, concentration-dependent phosphate uptake | [67] |
| S. vacuolatus | GBH (Glifosato Atanor) with 2.5% of the surfactant (alkyl aryl polyglycol ether) | 0–8 mg GLY/l | 96 h | growth, morphology, oxidative stress parameters | 96 h IC50 = 4.9 mg/L, metabolic and morphological changes (≥ 4 mg/L), oxidative damage (≥ 6 mg/L) | [68] |
| cyanobacterial species | pesticide adjuvants | dilution series | 96 h | growth inhibition | substance- and species-specific effects | [71] |
| N. microcarpa var. wrightii | technical-grade GLY, GBH (Roundup), AMPA | GLY, Roundup: 0.28, 3.5, 6 mg/L; AMPA: 0.03 mg/L | 7 d | photosynthetic rate, dark respiration rate, chl-a | higher toxicity of Roundup, stimulatory effect of AMPA | [80] |
| P. subcapitata | POEA | dilution series | 96 h | growth inhibition | 96 h EC50 = 4.1–4.9 mg/L | [81] |
| P. subcapitata C. vulgaris, Oophila sp | MON 0818 | dilution series | 96 h | growth inhibition | 96 h EC50 = 0.21–1.61 mg/L | [82] |
| P. subcapitata | APG k | dilution series | 72 h | growth inhibition | negligible aquatic toxicity | [83] |
| P. subcapitata | APG | dilution series | 72 h | growth inhibition | toxicity affected by the length of the carbon chain | [84] |
| green microalgae species | APG | 0.26–6.8 mg/L | 72 h | growth inhibition | 72 h EC50 = 0.32–2.7 mg/L | [85] |
| M. aeruginosa | GLY, Roundup | 0.06–29.6 µg/L | 21 d | cell number, chl-a, APA l activity | increased cell number and chl-a, inhibition (> 5.92 µg/L), GLY increased photosynthesis, concentration-dependent APA activity | [90] |
| freshwater microalgae | GLY | maximum tested concentration: 5.07 g/L | 80 min | chl-a fluorescence, cell viability | concentration-specific effect on maximum quantum yield of PSII m (< 0.17 mg/L) | [95] |
| microalgal and cyanobacterial species | Factor 540R | 10–1000 µg/L | 48 h | growth inhibition, photosynthetic parameters | 48 h EC50 = 406–724 µg/L, modified photosynthetic response (≥ 10 µg/L) | [96] |
2. Materials and Methods
2.1. Standard and Reagents
2.2. Selected Algae Monocultures
2.3. Algal Growth Inhibition Tests
2.4. Photosynthetic Activity Tests
2.5. Statistical Analysis
3. Results
3.1. Individual and Combined Effects on Algal Growth
3.2. Effects on the Photosynthetic Activity of Green Algae Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Solomon, K.R.; Thompson, D.G. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. J. Toxicol. Environ. Health Part B 2003, 6, 289–324. [Google Scholar] [CrossRef] [PubMed]
- Hanke, I.; Wittmer, I.; Bischofberger, S.; Stamm, C.; Singer, H. Relevance of urban glyphosate use for surface water quality. Chemosphere 2010, 81, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef]
- Gower, S.A.; Loux, M.M.; Cardina, J.; Harrison, S.K. Effect of planting date, residual herbicide, and postemergence application timing on weed control and grain yield in glyphosate-tolerant corn (Zea mays). Weed Technol. 2002, 16, 488–494. [Google Scholar] [CrossRef]
- Whigham, D.K.; Stoller, E.W. Soybean desiccation by paraquat, glyphosate, and ametryn to accelerate harvest. Agron. J. 1979, 71, 630–633. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing council directives 79/117/EEC and 91/414/EEC. OJ EU 2009, L309, 1–50. Available online: https://eur-lex.europa.eu/eli/reg/2009/1107/oj (accessed on 21 March 2024).
- European Commission. Commission Implementing Regulation (EU) 2023/2660 of 28 November 2023 renewing the approval of the active substance glyphosate in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) No 540/2011. OJ EU 2023, L2023/2660. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L_202302660&qid=1710855398897 (accessed on 21 March 2024).
- Defarge, N.; Takács, E.; Lozano, V.L.; Mesnage, R.; Vendômois, J.S.; Seralini, G.E.; Székács, A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Pub. Health 2016, 13, 264. [Google Scholar] [CrossRef]
- Travlos, I.; Cheimona, N.; Bilalis, D. Glyphosate efficacy of different salt formulations and adjuvant additives on various weeds. Agronomy 2017, 7, 60. [Google Scholar] [CrossRef]
- Foy, C. Adjuvants: Terminology, classification, and mode of action. In Adjuvants and Agrochemicals; Chow, P., Grant, C., Hinshalwood, A., Simundson, E., Eds.; CRC Press: Boca Raton, FL, USA, 1987; pp. 1–15. [Google Scholar]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Székács, A. Mechanism-related teratogenic, hormone modulant and other toxicological effects of veterinary and agricultural surfactants. Insights Vet. Sci. 2017, 1, 24–31. [Google Scholar] [CrossRef]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2016/1313 of 1 August 2016 amending Implementation Regulation (EU) No 540/2011 as regards the conditions of approval of the active substance glyphosate. OJ EU 2016, L208, 1–3. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.208.01.0001.01.ENG (accessed on 21 March 2024).
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring pesticide residues in surface and ground water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef]
- Klátyik, S.; Simon, G.; Oláh, M.; Takács, E.; Mesnage, R.; Antoniou, M.N.; Zaller, J.G.; Székács, A. Aquatic ecotoxicity of glyphosate, its formulations, and co-formulants: Evidence from 2010 to 2023. Environ. Sci. Eur. 2024, 36, 22. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Hébert, M.-P.; Fugère, V.; Gonzalez, A. The overlooked impact of rising glyphosate use on phosphorus loading in agricultural watersheds. Front. Ecol. Environ. 2019, 17, 48–56. [Google Scholar] [CrossRef]
- Zaller, J.G.; Weber, M.; Maderthaner, M.; Gruber, E.; Takács, E.; Mörtl, M.; Klátyik, S.; Győri, J.; Römbke, J.; Leisch, F.; et al. Effects of glyphosate-based herbicides and their active ingredients on earthworms, water infiltration and glyphosate leaching are influenced by soil properties. Environ. Sci. Eur. 2021, 33, 51. [Google Scholar] [CrossRef]
- Kjær, J.; Olsen, P.; Ullum, M.; Grant, R. Leaching of glyphosate and amino-methylphosphonic acid from Danish agricultural field sites. J. Environ. Qual. 2005, 34, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Huhn, C. More and enhanced glyphosate analysis is needed. Anal. Bioanal. Chem. 2018, 410, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Székács, A.; Darvas, B. Re-registration challenges of glyphosate in the European Union. Front. Environ. Sci. 2018, 6, 78. [Google Scholar] [CrossRef]
- Villeneuve, A.; Larroudé, S.; Humbert, J.F. Herbicide contamination of freshwater ecosystems: Impact on microbial communities. In Pesticides–Formulations, Effects, Fate; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 285–312. [Google Scholar]
- Chang, F.C.; Simcik, M.F.; Capel, P.D. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ. Toxicol. Chem. 2011, 30, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Montanarella, L.; Jones, A.; Fernández-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Lutri, V.F.; Matteoda, E.; Blarasin, M.; Aparicio, V.; Giacobone, D.; Maldonado, L.; Becher Quinodoz, F.; Cabrera, A.; Giuliano Albo, J. Hydrogeological features affecting spatial distribution of glyphosate and AMPA in groundwater and surface water in an agroecosystem. Córdoba, Argentina. Sci. Total Environ. 2020, 711, 134557. [Google Scholar] [CrossRef]
- Grandcoin, A.; Piel, S.; Baurès, E. AminoMethylPhosphonic acid (AMPA) in natural waters: Its sources, behavior and environmental fate. Weed Res. 2017, 117, 187–197. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Common Weed Killer Is Widespread in the Environment, U.S. Geological Survey. Available online: https://www.usgs.gov/programs/environmental-health-program/science/common-weed-killer-widespread-environment (accessed on 21 March 2024).
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Mörtl, M.; Németh, G.; Juracsek, J.; Darvas, B.; Kamp, L.; Rubio, F.; Székács, A. Determination of glyphosate residues in Hungarian water samples by immunoassay. Microchem. J. 2013, 107, 143–151. [Google Scholar] [CrossRef]
- Bonansea, R.I.; Filippi, I.; Wunderlin, D.A.; Marino, D.J.G.; Amé, M.V. The fate of glyphosate and AMPA in a freshwater endorheic basin: An ecotoxicological risk assessment. Toxics 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Caprile, A.C.; Aparicio, V.; Sasal, C.; Andriulo, E. Variation in glyphosate and AMPA concentrations of surface water and groundwater. Geophys. Res. Abstr. 2017, 19, EGU2017-2068. Available online: https://meetingorganizer.copernicus.org/EGU2017/EGU2017-2068.pdf (accessed on 21 March 2024).
- Centrum voor Milieuwetenschappen Leiden. Atlas Bestrijdingsmiddelen in Oppervlaktewater, Universiteit Leiden, Leiden, Netherlands. Available online: https://www.bestrijdingsmiddelenatlas.nl/atlas/1/1 (accessed on 21 March 2024).
- Poiger, T.; Buerge, I.J.; Bächli, A.; Müller, M.D.; Balmer, M.E. Occurrence of the herbicide glyphosate and its metabolite AMPA in surface waters in Switzerland determined with on-line solid phase extraction LC-MS/MS. Environ. Sci. Pollut. Res. 2017, 24, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Di Guardo, A.; Finizio, A. A new methodology to identify surface water bodies at risk by using pesticide monitoring data: The glyphosate case study in Lombardy Region (Italy). Sci. Total Environ. 2018, 610–611, 421–429. [Google Scholar] [CrossRef]
- Turner, J.A. The Pesticide Manual, 19th ed.; The British Crop Protection Council: Brighton, UK, 2021. [Google Scholar]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public Health. 2020, 15, 7519. [Google Scholar] [CrossRef]
- Klátyik, S.; Simon, G.; Oláh, M.; Mesnage, R.; Antoniou, M.N.; Zaller, J.G.; Székács, A. Terrestrial ecotoxicity of glyphosate, its formulations, and co-formulants: Evidence from 2010–2023. Environ. Sci. Eur. 2023, 35, 51. [Google Scholar] [CrossRef]
- Tush, D.; Meyer, M.T. Polyoxyethylene tallow amine, a glyphosate formulation adjuvant: Soil adsorption characteristics, degradation profile, and occurrence on selected soils from agricultural fields in Iowa, Illinois, Indiana, Kansas, Mississippi, and Missouri. Environ. Sci. Technol. 2016, 50, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Tush, D.; Maksimowicz, M.M.; Meyer, M.T. Dissipation of polyoxyethylene tallow amine (POEA) and glyphosate in an agricultural field and their co-occurrence on streambed sediments. Sci. Total Environ. 2018, 636, 212–219. [Google Scholar] [CrossRef]
- Morrás, H.; Behrends Kraemer, F.; Sainz, D.; Fernández, P.; Chagas, C. Soil structure and glyphosate fate under no-till management in the Pampa region. II. Glyphosate and AMPA persistence and spatial distribution in the long-term. A conceptual model. Soil Tillage Res. 2022, 223, 105471. [Google Scholar] [CrossRef]
- Green, J.M.; Beestman, G.B. Recently patented and commercialized formulation and adjuvant technology. Crop Prot. 2007, 26, 320–327. [Google Scholar] [CrossRef]
- Geetha, D.; Tyagi, R. Alkyl poly glucosides (APGs) surfactants and their properties: A review. Tenside Surfactants Deterg. 2012, 49, 417–427. [Google Scholar] [CrossRef]
- Rastogi, R. Fate of alkyl polyglucosides in the environment. J. Cosmet. Sci. 2021, 72, 91–98. [Google Scholar] [PubMed]
- Evalen, P.S.; Barnhardt, E.N.; Ryu, J.; Stahlschmidt, Z.R. Toxicity of glyphosate to animals: A meta-analytical approach, Environ. Pollut. 2024, 347, 123669. [Google Scholar] [CrossRef] [PubMed]
- Dabney, B.L.; Patiño, R. Low-dose stimulation of growth of the harmful alga, Prymnesium parvum, by glyphosate and glyphosate-based herbicides. Harmful Algae 2018, 80, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Asselborn, V.; Parodi, E.R. Toxic effects of chlorpyrifos, cypermethrin and glyphosate on the non-target organism Selenastrum capricornutum (Chlorophyta). An. Acad. Bras. Cienc. 2021, 93, e20200233. [Google Scholar] [CrossRef] [PubMed]
- Reno, U.; Doyle, S.R.; Momo, F.R.; Regaldo, L.; Gagneten, A.M. Effects of glyphosate formulations on the population dynamics of two freshwater cladoceran species. Ecotoxicology 2018, 27, 784–793. [Google Scholar] [CrossRef]
- Iummato, M.M.; Sabatini, S.E.; Cacciatore, L.C.; Cochón, A.C.; Cataldo, D.; de Molina, M.D.C.R.; Juárez, Á.B. Biochemical responses of the golden mussel Limnoperna fortunei under dietary glyphosate exposure. Ecotoxicol. Environ. Saf. 2018, 163, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, E.; Sehonova, P.; Plhalova, L.; Blahova, J.; Svobodova, Z.; Faggio, C. Effects of glyphosate on early life stages: Comparison between Cyprinus carpio and Danio rerio. Environ. Sci. Pollut. Res. 2018, 25, 8542–8549. [Google Scholar] [CrossRef]
- Bach, N.C.; Marino, D.J.G.; Natale, G.S.; Somoza, G.M. Effects of glyphosate and its commercial formulation, Roundup® Ultramax, on liver histology of tadpoles of the neotropical frog, Leptodactylus latrans (amphibia: Anura). Chemosphere 2018, 202, 289–297. [Google Scholar] [CrossRef]
- Schaffer, J.D.; Sebetich, M.J. Effects of aquatic herbicides on primary productivity of phytoplankton in the laboratory. Bull. Environ. Contam. Toxicol. 2004, 72, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, K.; Krishna Prasad, M.; Mohan Narasimha Rao, G. Algae in fresh water ecosystem. Phykos 2016, 46, 25–31. [Google Scholar]
- Maguire, R.J.; Wong, P.T.S.; Rhamey, J.S. Accumulation and metabolism of tri-n-butyltin cation by a green alga, Ankistrodesmus falcatus. Can. J. Fish. Aquat. 1984, 41, 537–540. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Whitton, B.A.; Higgins, S.N.; Paerl, H.W.; Brooks, B.W.; Wehr, J.D. Harmful algal blooms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Kociolek, P., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 873–920. [Google Scholar]
- Wu, N.; Dong, X.; Liu, Y.; Wang, C.; Baattrup-Pederson, A.; Riis, T. Using river microalgae as indicators for freshwater biomonitoring: Review of published research and future directions. Ecol. Indic. 2017, 81, 124–131. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Ravishankar, G.A. Algae-based bioremediation: Bioproducts and biofuels for biobusiness. In Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 457–493. [Google Scholar]
- Qu, M.; Wang, L.; Xu, Q.; An, J.; Mei, Y.; Liu, G. Influence of glyphosate and its metabolite aminomethylphosphonic acid on aquatic plants in different ecological niches. Ecotoxicol. Environ. Saf. 2022, 246, 114155. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, C.I.; Martínez-Jerónimo, F. Multistressor negative effects on an experimental phytoplankton community. The case of glyphosate and one toxigenic cyanobacterium on Chlorophycean microalgae. Sci. Total Environ. 2020, 717, 137186. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qiu, Z.; Zhou, Y.; Du, Y.; Liu, C.; Ye, J.; Hu, X. Physiological effects of the herbicide glyphosate on the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2016, 178, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, D.; Lipok, J. Glyphosate dose modulates the uptake of inorganic phosphate by freshwater cyanobacteria. J. Appl. Phycol. 2018, 30, 299–309. [Google Scholar] [CrossRef]
- Iummato, M.M.; Fassiano, A.; Graziano, M.; Dos Santos Afonso, M.; Ríos de Molina, M.D.C.; Juárez, Á.B. Effect of glyphosate on the growth, morphology, ultrastructure and metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 15, 471–479. [Google Scholar] [CrossRef]
- Smedbol, É.; Gomes, M.P.; Paquet, S.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Effects of low concentrations of glyphosate-based herbicide Factor 540® on an agricultural stream freshwater phytoplankton community. Chemosphere 2018, 192, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, X.; Li, L.; Lin, S. Differential growth responses of marine phytoplankton to herbicide glyphosate. PLoS ONE 2016, 11, e0151633. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qin, W.; Lu, N.; Wang, P.; Huang, C.; Xu, R. Differential sensitivity of three cyanobacteria (Anabaena flos-aquae, Microcystis flos-aquae and Mirocystis aeruginosa to 10 pesticide adjuvants. Bull. Environ. Contam. Toxicol. 2005, 75, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Arunakumara, K.; Walpola, B.; Yoon, M. Metabolism and degradation of glyphosate in aquatic cyanobacteria: A review. Afr. J. Microbiol. Res. 2013, 7, 4084–4090. [Google Scholar]
- EGEIS Aquatic Ecotoxicity of Glyphosate and Formulated Products Containing Glyphosate. European Glyphosate Environmental Information Sources. Available online: http://www.egeis.org/cd-info/Aquatic-ecotoxicity-of-glyphosate-and-formulated-products-containing-glyphosate.pdf (accessed on 21 March 2024).
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk. Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- MacBean, C. The Pesticide Manual, 16th ed.; The British Crop Protection Council: Brighton, UK, 2012. [Google Scholar]
- Gonzalez, D.; Juárez, A.; Krug, C.; Santos, M.; Vera, S. Freshwater periphyton response to technical-grade and two commercial formulations of glyphosate. Ecol. Austral. 2019, 29, 020–027. [Google Scholar] [CrossRef]
- Vera, M.S.; Trinelli, M.A. First evaluation of the periphyton recovery after glyphosate exposure. Environ. Pollut. 2021, 290, 117998. [Google Scholar] [CrossRef] [PubMed]
- Bricheux, G.; Le Moal, G.; Hennequin, C.; Coffe, G.; Donnadieu, F.; Portelli, C.; Bohatier, J.; Forestier, C. Characterization and evolution of natural aquatic biofilm communities exposed in vitro to herbicides. Ecotoxicol. Environ. Saf. 2013, 88, 126–134. [Google Scholar] [CrossRef]
- de Campos Oliveira, R.; Boas, L.K.V.; Branco, C.C.Z. Assessment of the potential toxicity of glyphosate-based herbicides on the photosynthesis of Nitella microcarpa var. wrightii (Charophyceae). Phycologia 2016, 55, 577–584. [Google Scholar] [CrossRef]
- van Ginkel, C.G.; Stroo, C.A.; Kroon, A.G.M. Biodegradability of ethoxylated fatty amines and amides and the non-toxicity of their biodegradation products. Tenside Surfactant. Deterg. 1993, 30, 213–216. [Google Scholar] [CrossRef]
- Rodriguez-Gil, J.L.; Prosser, R.; Poirier, D.; Lissemore, L.; Thompson, D.; Hanson, M.; Solomon, K.R. Aquatic hazard assessment of MON 0818, a commercial mixture of alkylamine ethoxylates commonly used in glyphosate-containing herbicide formulations. Part 1: Species sensitivity distribution from laboratory acute exposures. Environ. Toxicol. Chem. 2017, 36, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Petersen, G.; Seiero, C.; Torslov, J. Biodegradability and aquatic toxicity of glycoside surfactants and a nonionic alcohol ethoxylate. J. Am. Oil Chem. Soc. 1996, 73, 929–933. [Google Scholar] [CrossRef]
- Jurado, E.; Fernández-Serrano, M.; Núnez-Olea, J.; Lechuga, M.; Jiménez, J.L.; Ríos, F. Acute toxicity of alkylpolyglucosides to Vibrio fischeri, Daphnia magna and microalgae: A comparative study. Bull. Environ. Contam. Toxicol. 2012, 88, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, Z.; Vidakovic-Cifrek, Z.; Puntaric, D. Toxicity of surfactants to green microalgae Pseudokirchneriella subcapitata and Scenedesmus subspicatus and to marine diatoms Phaeodactylum tricornutum and Skeletonema costatum. Chemosphere 2005, 61, 1061–1068. [Google Scholar] [CrossRef]
- Kautsky, H.; Hirsch, A. Neue Versuche zur Kohlenstoffassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology II: Interpretation of fluorescence signal. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef]
- Barócsi, A.; Kocsányi, L.; Várkonyi, S.; Richter, P.; Csintalan, Z.; Szente, K. Two-wavelength, multipurpose, truly portable chlorophyll fluorometer and its application in field monitoring of phytoremediation. Meas. Sci. Technol. 2000, 11, 717–729. [Google Scholar] [CrossRef]
- Lenk, S.; Gádoros, P.; Kocsányi, L.; Barócsi, A. Teaching laser-induced fluorescence of plant leaves. Eur. J. Phys. 2016, 37, 064003. [Google Scholar] [CrossRef]
- Qiu, H.; Geng, J.; Ren, H.; Xia, X.; Wang, X.; Yu, Y. Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J. Hazard. Mater. 2013, 248–249, 172–176. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Temperature and light modulation of herbicide toxicity on algal and cyanobacterial physiology. Front. Environ. Sci. 2017, 5, 50. [Google Scholar] [CrossRef]
- Cobb, A.H.; Reade, J.P.H. Harmful algal blooms. In Herbicides and Plant Physiology; Cobb, A.H., Reade, J.P.H., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 176–197. [Google Scholar]
- Gomes, M.P.; Le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-dependent inhibition of photosynthesis in willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Le Manac’h, S.G.; Maccario, S.; Labrecque, M.; Lucotte, M.; Juneau, P. Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic. Biochem. Physiol. 2016, 130, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.J.; Berges, J.A.; Young, E.B. Rapid effects of diverse toxic water pollutants on chlorophyll a fluorescence: Variable responses among freshwater microalgae. Weed Res. 2012, 46, 2615–2626. [Google Scholar] [CrossRef] [PubMed]
- Smedbol, É.; Lucotte, M.; Labrecque, M.; Lepage, L.; Juneau, P. Phytoplankton growth and PSII efficiency sensitivity to a glyphosate-based herbicide (Factor 540®). Aquat. Toxicol. 2017, 192, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Monsanto Europe S.A. Material Safety Data Sheet of Roundup Classic. Monsanto Europe S.A./N.V.: Antwerp, Belgium, 2015. [Google Scholar]
- Syngenta. Material Safety Data Sheet of Medallon Premium; Syngenta Magyarország Kft.: Budapest, Hungary, 2018. [Google Scholar]
- Scandinavian Culture Collection of Algae and Protozoa: Media Recipes. Available online: https://www.sccap.dk/media/ (accessed on 21 March 2024).
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- ISO 8692:2012; Water Quality-Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. International Organization for Standardization: Geneva, Switzerland, 2012.
- Organisation for Economic Co-operation and Development Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Publishing, Paris. Available online: https://read.oecd-ilibrary.org/environment/test-no-201-alga-growth-inhibition-test_9789264069923-en#page1 (accessed on 21 March 2024).
- Stevenson, R.J.; Lowe, R.L. Sampling and interpretation of algal patterns for water quality assessment. In Rationale for Sampling and Interpretation of Ecological Data in the Assessment of Freshwater Ecosystems; Isom, R.G., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 1986; pp. 118–149. [Google Scholar]
- McCormick, P.V.; Cairns, J. Algae as indicators of environmental change. J. Appl. Phycol. 1994, 6, 509–526. [Google Scholar] [CrossRef]
- ISO 10260:1992; Water Quality-Measurement of Biochemical Parameters–Spectrometric Determination of the Chlorophyll-a Concentration. International Organization for Standardization: Geneva, Switzerland, 1992.
- Lázár, D.; Takács, E.; Mörtl, M.; Klátyik, S.; Barócsi, A.; Kocsányi, L.; Lenk, S.; Domján, L.; Szarvas, G.; Lengyel, E.; et al. Application of a fluorescence-based instrument prototype for chlorophyll measurements and its utility in an herbicide algal ecotoxicity assay. Water 2023, 15, 1866. [Google Scholar] [CrossRef]
- Barócsi, A.; Lenk, S.; Kocsányi, L.; Buschmann, C. Excitation kinetics during induction of chlorophyll a fluorescence. Photosynthetica 2009, 47, 104–111. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Brain, P.; Cousens, R. An equation to describe dose responses where there is stimulation of growth at low doses. Weed Res. 1989, 29, 93–96. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Jensen, S.M.; Gerhard, D.; Streibig, J.C. A hormesis effect on lettuce growth. In Dose-Response Analysis Using R; Ritz, C., Jensen, S.M., Gerhard, D., Streibig, J.C., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 23–26. [Google Scholar]
- Wong, P.K. Effect of 2,4-D, glyphosate and paraquat on growth, photosynthesis and chlorophyll-a synthesis of Scenedesmus quadricauda Berb 614. Chemosphere 2000, 41, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J. A mechanistic analysis of penetration of glyphosate salts across astomatous cuticular membranes. Pest Manag. Sci. 2022, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Ewacha, M.V.A.; Goldsborough, L.G. The response of Scenedesmus quadricauda and Selenastrum capricornutum to glyphosate toxicity (Roundup® formulation) with cellular growth and chlorophyll-a synthesis as endpoints. Proc. Manitoba’s Undergrad. Sci. Eng. Res. 2013, 1, 1–19. [Google Scholar]
- Powell, H.A.; Kerby, N.W.; Rowell, P. Natural tolerance of cyanobacteria to the herbicide glyphosate. New Phytol. 1991, 119, 421–426. [Google Scholar] [CrossRef]
- Sáenz, M.E.; Di Marzio, W. Ecotoxicity of herbicide glyphosate to four chlorophyceaen freshwater algae. Limnetica 2009, 28, 149–158. [Google Scholar] [CrossRef]
- LISEC Alga, growth inhibition test. Effect of MON 2139 on the growth of Selenastrum capricomutum. In Monsanto Unpublished Study XX-89-093; LISEC, Study Centre for Ecology and Forestry: Bokrijk, Belgium, 1989. [Google Scholar]
- Cedergreen, N.; Streibig, J.C. The toxicity of herbicides to non-target aquatic plants and algae: Assessment of predictive factors and hazard. Pest Manag. Sci. 2005, 61, 1152–1160. [Google Scholar] [CrossRef]
- Pereira, J.; Antunes, S.C.; Castro, B.B.; Marques, C.R.; Goncalves, A.M.M.; Goncalves, F.; Pereira, R. Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: Commercial formulation versus active ingredient. Ecotoxicology 2009, 18, 455–463. [Google Scholar] [CrossRef]
- Lipok, J.; Studnik, H.; Gruyaert, S. The toxicity of Roundup 360 SL formulation and its main constituents: Glyphosate and isopropylamine towards non-target water photoautotrophs. Ecotoxicol. Environ. Saf. 2010, 73, 1681–1688. [Google Scholar] [CrossRef]
- Steber, J.; Guhl, W.; Stelter, N.; Schroder, F.R. Alkyl polyglycosides -ecological evaluation of a new generation of nonionic surfactants. Tenside Surfactants Deterg. 1995, 32, 515–521. [Google Scholar] [CrossRef]
- Gomes, M.P.; Juneau, P. Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: Is the mitochondrial electron transport chain a target of this herbicide? Environ. Pollut. 2016, 218, 402–409. [Google Scholar] [CrossRef]
- Rasch, A.; Hunsche, M.; Mail, M.; Burkhardt, J.; Noga, G.; Pariyar, S. Agricultural adjuvants may impair leaf transpiration and photosynthetic activity. Plant Physiol. Biochem. 2018, 132, 229–237. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Cutler, G.C. Insecticide induced hormesis and arthropod pest management. Pest Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Kendig, E.L.; Le, H.H.; Belcher, S.M. Defining hormesis: Evaluation of a complex concentration response phenomenon. Int. J. Toxicol. 2010, 29, 235–246. [Google Scholar] [CrossRef]
- Bakonyi, G.; Szabó, B.; Seres, A. A hormézis, mint ökotoxikológiai jelenség, különös tekintettel a növényvédelemre. bioKontroll 2017, 2, 47–53. (In Hungarian) [Google Scholar]
- Calabrese, E.J. Overcompensation stimulation: A mechanism for hormetic effects. Critical Rev. Toxicol. 2001, 31, 425–470. [Google Scholar] [CrossRef]
- Jager, T.; Barsi, A.; Ducrot, V. Hormesis on life-history traits: Is there such thing as a free lunch? Ecotoxicology 2013, 22, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Forbes, V.E. Is hormesis an evolutionary expectation? Functional Ecol. 2000, 14, 12–24. [Google Scholar] [CrossRef]
- Saxton, M.A.; Morrow, E.A.; Bourbonniere, R.A.; Wilhelm, S.W. Glyphosate influence on phytoplankton community structure in Lake Erie. J. Great Lakes Res. 2011, 37, 683–690. [Google Scholar] [CrossRef]
- Vera, M.S.; Lagomarsino, L.; Sylvester, M.; Pérez, G.L.; Rodriguez, P.; Mugni, H.; Sinistro, R.; Ferraro, M.; Bonetto, C.; Zagares, H.; et al. New evidences of Roundup® (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trinidade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Panizzi, S.; Suciu, N.A.; Trevisan, M. Combined ecotoxicological risk assessment in the frame of European authorization of pesticides. Sci. Total Environ. 2017, 580, 136–146. [Google Scholar] [CrossRef]
- Lozano, V.L.; Pizarro, H.N. Glyphosate lessons: Is biodegradation of pesticides a harmless process for biodiversity? Environ. Sci. Eur. 2024, 36, 55. [Google Scholar] [CrossRef]
- Aparicio, V.C.; de Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Brausch, J.M.; Smith, P.N. Toxicity of three POEA surfactant formulations to the fairy shrimp Thamnocephalus platyurus. Arch. Environ. Contam. Toxicol. 2007, 52, 217–222. [Google Scholar] [CrossRef]
- Li, M.; Du, F.; Cao, C.; Li, B.; Zhai, X. Effect of glyphosate isopropylamine on the surface tension and surface dilational rheology properties of polyoxyethylene tallow amine surfactant. J. Dispers. Sci. Technol. 2016, 37, 213–221. [Google Scholar] [CrossRef]
- Mesnage, R.; Antoniou, M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticide. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef] [PubMed]
- Klátyik, S.; Takács, E.; Mörtl, M.; Földi, A.; Trábert, Z.; Ács, É.; Darvas, B.; Székács, A. Dissipation of the herbicide active ingredient glyphosate in natural water samples in the presence of biofilms. Int. J. Environ. Anal. Chem. 2017, 97, 901–921. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ EU 2013, L93, 85–151. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R0284&qid=1707814054061 (accessed on 21 March 2024).
- Seralini, G.-E. Pesticides in formulations: New revolutionary findings. Toxics 2024, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.M.; Triplett, G.B.; Kramer, R.M. A watershed study of glyphosate transport in runoff. J. Environ. Qual. 1980, 9, 661–665. [Google Scholar] [CrossRef]
- Servizi, J.A.; Gordon, R.W.; Martens, D.W. Acute toxicity of Garlon 4 and Roundup herbicides to salmon, Daphnia, and Trout. Bull. Environ. Contam. Toxicol. 1987, 39, 15–22. [Google Scholar] [CrossRef]
- Mensink, H.; Janssen, P. Glyphosate. 1994. Available online: http://www.inchem.org/documents/ehc/ehc/ehc159.htm (accessed on 9 February 2024).


| Active ingredient (AI) | |||||
| Chemical Name | CAS No.1 | Concentration of the AI | Physical Appearance | Chemical Structure | |
| glyphosate isopropylammonium (IPA) salt | 38641-94-0 | 62% (486 g/L glyphosate acid) | water-soluble emulsion |  | |
| Glyphosate-based formulations | |||||
| Product name | AI | Concentration of the AI | Co-formulants | Concentration of the co-formulants | Type of formulation |
| Roundup Classic | glyphosate IPA salt | 41.5% (360 g/L glyphosate acid) | mixture of polyethoxylated tallow amines (POEA) | 15.5% | liquid water-soluble concentrate |
| Medallon Premium | glyphosate diammonium salt(CAS 69254-40-6) | 34% (360 g/L glyphosate acid) | alkyl polyglucosides (APG) | 10–20% | liquid water-soluble concentrate |
| Co-formulants | |||||
| Product name | Co-formulant | Concentration of the co-formulant | Additives | Type of formulation | Chemical structure |
| Emulson AG GPE 3SS | POEA (CAS 61791-26-2) | 100% | – | water-soluble emulsion |  |
| Plantapon LGC | APG (Na-lauryl glucose carboxylate CAS 383178-66-3 + lauryl glucoside CAS 110615-47-9) | 28.5–34.0% | water: 66–71.5% | water-soluble emulsion |  |
| Fluorescence Parameter | Definition | Interpretation |
|---|---|---|
| Fo | observed | Non-variable (original) fluorescence intensity |
| Fp | observed | Peak fluorescence intensity, maximum fluorescence at a non-saturating light pulse |
| Fv* | Fp–Fo | Variable fluorescence in terms of Fp |
| Fv*/Fp | Fv*/Fp | Proxy of quantum efficiency of photosystem II |
| Fs | observed | Steady-state (terminal) fluorescence |
| Fd | Fp–Fs | Fluorescence decrease in terms of Fp |
| Rfd* | Fd/Fs | Fluorescence decrease ratio |
| 72 h EC50 Values (mg/L) 1 | ||||
|---|---|---|---|---|
| Algae Species | GLY | Roundup Classic 2 | POEA | |
| GLY cont. | POEA cont. | |||
| Pseudokirchneriella subcapitata | 125.2 ± 16.5 | 12.2 ± 3.1 | 2.6 ± 0.7 | |
| 5.1 ± 1.3 | 1.9 ± 0.5 | |||
| Desmodesmus subspicatus | 132.9 ± 2.3 | 34.0 ± 6.9 | 4.4 ± 0.4 | |
| 14.1 ± 2.9 | 5.3 ± 1.1 | |||
| Scenedesmus obtusiusculus | 73.1 ± 21.2 | 65.8 ± 9.0 | 6.9 ± 1.6 | |
| 27.3 ± 3.7 | 10.2 ± 1.4 | |||
| 72 h EC50 Values (mg/L) 1 | ||||
|---|---|---|---|---|
| Algae Species | GLY | Roundup Classic 2 | POEA | |
| GLY cont. | POEA cont. | |||
| Pseudokirchneriella subcapitata | 105.3 ± 17.8 | 34.9 ± 3.2 | 1.9 ± 0.3 | |
| 14.5 ± 1.36 | 5.4 ± 0.5 | |||
| Desmodesmus subspicatus | 73.8 ± 5.3 | 32.3 ± 9.2 | 4.9 ± 0.6 | |
| 13.4 ± 3.8 | 5.0 ± 1.4 | |||
| Scenedesmus obtusiusculus | 51.1 ± 2.6 | 25.4 ± 8.5 | 4.4 ± 0.9 | |
| 10.5 ± 3.5 | 3.9 ± 0.6 | |||
| Anabaena flos-aquae | 17.4 ± 6.0 | n.m. 3 | n.m. | |
| n.m. | n.m. | |||
| 72 h EC50 Values (mg/L) 1 | ||||
|---|---|---|---|---|
| Algae Species | GLY | Medallon Premium 2 | APG | |
| GLY cont. | APG cont. | |||
| Pseudokirchneriella subcapitata | 125.2 ± 16.5 | 125.7 ± 13.7 | 23.0 ± 2.3 | |
| 42.7 ± 4.7 | 18.9 ± 2.1 | |||
| Desmodesmus subspicatus | 132.9 ± 2.3 | 720.9 ± 96.6 | 64.3 ± 12.9 | |
| 245.1 ± 32.8 | 108.1 ± 14.5 | |||
| Scenedesmus obtusiusculus | 73.1 ± 21.2 | 687.5 ± 171.9 | 137.9 ± 19.1 | |
| 233.8 ± 58.4 | 103.1 ± 25.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klátyik, S.; Takács, E.; Barócsi, A.; Lenk, S.; Kocsányi, L.; Darvas, B.; Székács, A. Hormesis, the Individual and Combined Phytotoxicity of the Components of Glyphosate-Based Formulations on Algal Growth and Photosynthetic Activity. Toxics 2024, 12, 257. https://doi.org/10.3390/toxics12040257
Klátyik S, Takács E, Barócsi A, Lenk S, Kocsányi L, Darvas B, Székács A. Hormesis, the Individual and Combined Phytotoxicity of the Components of Glyphosate-Based Formulations on Algal Growth and Photosynthetic Activity. Toxics. 2024; 12(4):257. https://doi.org/10.3390/toxics12040257
Chicago/Turabian StyleKlátyik, Szandra, Eszter Takács, Attila Barócsi, Sándor Lenk, László Kocsányi, Béla Darvas, and András Székács. 2024. "Hormesis, the Individual and Combined Phytotoxicity of the Components of Glyphosate-Based Formulations on Algal Growth and Photosynthetic Activity" Toxics 12, no. 4: 257. https://doi.org/10.3390/toxics12040257
APA StyleKlátyik, S., Takács, E., Barócsi, A., Lenk, S., Kocsányi, L., Darvas, B., & Székács, A. (2024). Hormesis, the Individual and Combined Phytotoxicity of the Components of Glyphosate-Based Formulations on Algal Growth and Photosynthetic Activity. Toxics, 12(4), 257. https://doi.org/10.3390/toxics12040257