Decreased Ubiquitination and Acetylation of Histones 3 and 4 Are Associated with Obesity-Induced Disorders of Spermatogenesis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Treatments
2.2. Body Composition Analysis
2.3. Measurement of Serum Lipid Levels
2.4. Testis Index and Histology
2.5. Immunohistochemistry (IHC) Assay
2.6. Sperm Count, Motility, and Malformation
2.7. Transmission Electron Microscopy (TEM)
2.8. Reverse Transcription PCR (RT-PCR)
2.9. Western Blots
2.10. Statistical Analysis
3. Results
3.1. Establishment of a Model for Obese Male Mice
3.2. The Serum Lipid Contents Were Altered in Mice with HFD-Induced Obesity
3.3. The Fertility Was Decreased in Mice with HFD-Induced Obesity
3.4. The Testes Were Damaged in Mice with HFD-Induced Obesity
3.5. The Sperm Quality Was Lower in Mice with HFD-Induced Obesity
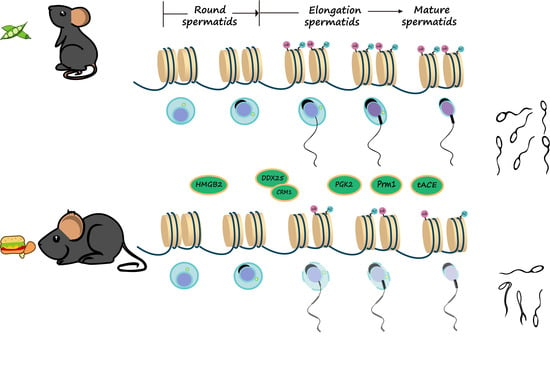
3.6. The mRNA and Protein Levels of Genes Associated with Spermatogenesis Were Decreased in Testes of Obese Mice
3.7. The Ubiquitination and Acetylation of Histones 3 and 4 in Testes Were Diminished in Male Mice with HFD-Induced Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 2017, 154, R123–R131. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.; Nemani, H.; Pothani, S.; Khambata, K.; Kumar, A.; Kallamadi, P.R.; Balasinor, N.H. Genetically Inherited Obesity and High-Fat Diet-Induced Obesity Differentially Alter Spermatogenesis in Adult Male Rats. Endocrinology 2019, 160, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Dieases, G.B. High body-mass index—Level 2 risk. Lancet 2020, 393, R2. [Google Scholar]
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.F.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.M.; Millar, K.; Jones, C.; Fatum, M.; Coward, K. Deleterious effects of obesity upon the hormonal and molecular mechanisms controlling spermatogenesis and male fertility. Hum. Fertil. 2015, 18, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, G.; Ila, V.; Dornbush, J.; Bernstein, A.; Loloi, J.; Pozzi, E.; Miller, D.; Ramasamy, R. Male obesity: Associated effects on fertility and the outcomes of offspring. Andrology 2023. online ahead of print. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yin, Y.; Wang, G.; Liu, Z.; Liu, L.; Sun, F. Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep. 2014, 4, 4260. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Mumford, S.L.; Chen, Z.; Browne, R.W.; Boyd Barr, D.; Kim, S.; Buck Louis, G.M. Lipid concentrations and semen quality: The LIFE study. Andrology 2014, 2, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, Z.; Ge, X.; Li, C.; Xue, M.; Liang, K.; Ma, R.; Ouyang, L.; Zheng, L.; Jing, J.; et al. LncRNA Tug1 maintains blood-testis barrier integrity by modulating Ccl2 expression in high-fat diet mice. Cell. Mol. Life Sci. 2022, 79, 114. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Liang, C.; Manthari, R.K.; Yu, Y.X.; Gao, Y.; Liu, Y.; Jiang, S.S.; Tikka, C.; Wang, J.D.; Zhang, J.H. Arsenic influences spermatogenesis by disorganizing the elongation of spermatids in adult male mice. Chemosphere 2020, 238, 124650. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Morris, C.-H.; Koh, E.; Sheng, Y.; Maeda, Y.; Gutti, R.; Namiki, M.; Dufau, M.L. Polymorphism of the GRTH/DDX25 gene in normal and infertile Japanese men: A missense mutation associated with loss of GRTH phosphorylation. Mol. Hum. Reprod. 2007, 13, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, R.; Dufau, M.L. Germ Cell Nuclear Factor (GCNF/RTR) Regulates Transcription of Gonadotropin-Regulated Testicular RNA Helicase (GRTH/DDX25) in Testicular Germ Cells--The Androgen Connection. Mol. Endocrinol. 2015, 29, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Villar, J.; Tsai-Morris, C.-H.; Dufau, M.L. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), a negative regulator of luteinizing/chorionic gonadotropin hormone-induced steroidogenesis in Leydig cells: Central role of steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 2011, 286, 29932–29940. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Hassan, S.A.; Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Characterization of the Phosphorylation Site of GRTH/DDX25 and Protein Kinase A Binding Interface Provides Structural Basis for the Design of a Non-Hormonal Male Contraceptive. Sci. Rep. 2019, 9, 6705. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gao, H.; Li, W.; Liu, C. Essential Role of Histone Replacement and Modifications in Male Fertility. Front. Genet. 2019, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Karahan, G.; Martel, J.; Rahimi, S.; Farag, M.; Matias, F.; MacFarlane, A.J.; Chan, D.; Trasler, J. Higher incidence of embryonic defects in mouse offspring conceived with assisted reproduction from fathers with sperm epimutations. Hum. Mol. Genet. 2023, 33, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.S.; Bera, P.; Khambata, K.; Balasinor, N.H. Paternal obesity induces epigenetic aberrations and gene expression changes in placenta and fetus. Mol. Reprod. Dev. 2023, 90, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; Ohlsson-Teague, E.M.; Print, C.G.; Sandeman, L.Y.; Lane, M. Sperm microRNA Content Is Altered in a Mouse Model of Male Obesity, but the Same Suite of microRNAs Are Not Altered in Offspring’s Sperm. PLoS ONE 2016, 11, e0166076. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.L.; Ni, F.D.; Yang, W.X. The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene 2019, 706, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Y.; Wu, J.; Ye, L.; Gavrilina, G.B.; Saunders, T.L.; Yu, X. RNF8-Dependent Histone Modifications Regulate Nucleosome Removal during Spermatogenesis. Dev. Cell 2010, 18, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.-T.; Kang, J.-Y.; Dai, P.; Wang, X.; Li, F.; Zhao, S.; Zhang, M.; Hua, M.-M.; Lu, Y.; Zhu, Y.; et al. Ubiquitination-Deficient Mutations in Human Piwi Cause Male Infertility by Impairing Histone-to-Protamine Exchange during Spermiogenesis. Cell 2017, 169, 1090–1104.e13. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Phillips, P.M.; Johnstone, A.F.M. A noninvasive method to study regulation of extracellular fluid volume in rats using nuclear magnetic resonance. Am. J. Physiol. Renal Physiol. 2016, 310, F426–F431. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Vassiliou, C.C.; Colucci, L.A.; Cima, M.J. (1)H nuclear magnetic resonance (NMR) as a tool to measure dehydration in mice. NMR Biomed. 2015, 28, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, Y.; Liang, C.; Shi, Y.; Zhu, Y.; Zheng, H.; Wang, J.; Zhang, J. Fluoride-induced unrestored arrest during haploid period of spermatogenesis via the regulation of DDX25 in rats. Environ. Pollut. 2019, 253, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Patti, M.E. Paternal Nongenetic Intergenerational Transmission of Metabolic Disease Risk. Curr. Diabetes Rep. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Sousa, M.; Silva, B.M.; Monteiro, M.P.; Alves, M.G. Obesity, energy balance and spermatogenesis. Reproduction 2017, 153, R173–R185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Guo, C.C.; Yu, Y.X.; Xie, L.; Chang, C.Q. Establishment of high-fat diet-induced obesity and insulin resistance model in rats. Beijing Da Xue Xue Bao Yi Xue Ban 2020, 52, 557–563. [Google Scholar] [CrossRef]
- Komninos, D.; Ramos, L.; van der Heijden, G.W.; Morrison, M.C.; Kleemann, R.; van Herwaarden, A.E.; Kiliaan, A.J.; Arnoldussen, I.A.C. High fat diet-induced obesity prolongs critical stages of the spermatogenic cycle in a Ldlr−/−.Leiden mouse model. Sci. Rep. 2022, 12, 430. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Messa, G.A.M.; Piasecki, M.; Hurst, J.; Hill, C.; Tallis, J.; Degens, H. The impact of a high-fat diet in mice is dependent on duration and age, and differs between muscles. J. Exp. Biol. 2020, 223, jeb217117. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ying, L.; Hong, G.; Wang, Y. The effects of the aqueous extract and residue of Matcha on the antioxidant status and lipid and glucose levels in mice fed a high-fat diet. Food Funct. 2016, 7, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Franssen, R.; Monajemi, H.; Stroes, E.S.G.; Kastelein, J.J.P. Obesity and dyslipidemia. Med. Clin. N. Am. 2011, 95, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Elías, M.D.; Rainero Cáceres, T.S.; Giaccagli, M.M.; Guazzone, V.A.; Dalton, G.N.; De Siervi, A.; Cuasnicú, P.S.; Cohen, D.J.; Da Ros, V.G. Association between high-fat diet feeding and male fertility in high reproductive performance mice. Sci. Rep. 2019, 9, 18546. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-F.; Feng, Q.; Ge, Z.-Y.; Guo, Y.; Zhou, F.; Zhang, K.-S.; Wang, X.-W.; Lu, W.-H.; Liang, X.-W.; Gu, Y.-Q. Obesity impairs male fertility through long-term effects on spermatogenesis. BMC Urol. 2018, 18, 42. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Lehti, M.S.; Sironen, A. Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 2016, 151, R43–R54. [Google Scholar] [CrossRef]
- Tsai-Morris, C.H.; Sheng, Y.; Lee, E.; Lei, K.J.; Dufau, M.L. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6373–6378. [Google Scholar] [CrossRef] [PubMed]
- Dufau, M.L.; Tsai-Morris, C.H. Gonadotropin-regulated testicular helicase (GRTH/DDX25): An essential regulator of spermatogenesis. Trends Endocrinol. Metab. TEM 2007, 18, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Rocak, S.; Linder, P. DEAD-box proteins: The driving forces behind RNA metabolism. Nat. Rev. Mol. Cell. Biol. 2004, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Pace, F.V. DExD/H box RNA helicases: Multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006, 34, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.E.; Hanson, E.S.; Capecchi, M.R. Sequence-independent assembly of spermatid mRNAs into messenger ribonucleoprotein particles. Mol. Cell. Biol. 1999, 19, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Tsai-Morris, C.H.; Gutti, R.; Maeda, Y.; Dufau, M.L. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J. Biol. Chem. 2006, 281, 35048–35056. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Kojima, K.; Hail, N., Jr.; Tabe, Y.; Andreeff, M. Expression, function, and targeting of the nuclear exporter chromosome region maintenance 1 (CRM1) protein. Pharmacol. Ther. 2015, 153, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.O. HMG1 and 2: Architectural DNA-binding proteins. Biochem. Soc. Trans. 2001, 29, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; McCarrey, J.R.; Hecht, N.B. A cytoplasmic variant of the KH-type splicing regulatory protein serves as a decay-promoting factor for phosphoglycerate kinase 2 mRNA in murine male germ cells. Nucleic Acids Res. 2008, 36, 7157–7167. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Geyer, C.B.; Hornecker, J.L.; Patel, K.T.; McCarrey, J.R. In vivo analysis of developmentally and evolutionarily dynamic protein-DNA interactions regulating transcription of the Pgk2 gene during mammalian spermatogenesis. Mol. Cell. Biol. 2007, 27, 7871–7885. [Google Scholar] [CrossRef] [PubMed]
- Ojaghi, M.; Kastelic, J.; Thundathil, J. Testis-specific isoform of angiotensin-converting enzyme (tACE) is involved in the regulation of bovine sperm capacitation. Mol. Reprod. Dev. 2017, 84, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Odroniec, A.; Olszewska, M.; Kurpisz, M. Epigenetic markers in the embryonal germ cell development and spermatogenesis. Basic Clin. Androl. 2023, 33, 6. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.G.; Carrell, D.T. The paternal epigenome and embryogenesis: Poising mechanisms for development. Asian J. Androl. 2011, 13, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, J.; Reynoird, N.; Montellier, E.; Boussouar, F.; Rousseaux, S.; Khochbin, S. From meiosis to postmeiotic events: The secrets of histone disappearance. FEBS J. 2010, 277, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Sonnack, V.; Failing, K.; Bergmann, M.; Steger, K. Expression of hyperacetylated histone H4 during normal and impaired human spermatogenesis. Andrologia 2002, 34, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Yelumalai, S.; Jones, C.; Coward, K. Aberrant protamine content in sperm and consequential implications for infertility treatment. Hum. Fertil. 2014, 17, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, X.; Tian, X.; Yang, D.; Dong, Y.; Chen, F.; Fang, X. The expression, function, and utilization of Protamine1: A literature review. Transl. Cancer Res. 2021, 10, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ji, S.-Y.; Busayavalasa, K.; Shao, J.; Yu, C. Meiosis I progression in spermatogenesis requires a type of testis-specific 20S core proteasome. Nat. Commun. 2019, 10, 3387. [Google Scholar] [CrossRef] [PubMed]






| Gene of Interest | Primer Sequence |
|---|---|
| DDX25 | F: 5′-ATGGCGTCGTTACTTTGGGG-3′ |
| R: 5′-AGAGCCGTCTATGTTTGGGAC-3′ | |
| CRM1 | F: 5′-CTGCTTGATTTCAGCCAAAAACT-3′ |
| R: 5′-GTATTTCGTGTTCATGTTCTGCG-3′ | |
| HMGB2 | F: 5′-AAGAGCGACAAAGCTCGTTATG-3′ |
| R: 5′-GCAGTATCTCCAATAGACAGGC-3′ | |
| PGK2 | F: 5′-CCACCTCCAATGGCTGTATC-3′ R: 5′-CTACCCCTGGAAGGGTTTTG-3′ |
| tACE | F: 5′-AGTATGACCGGACAGCCAAG-3′ R: 5′-CCAGGTGCCATATTTCAAGG-3′ |
| Prm1 | F: 5′- CCGTCGCAGACGAAGATGTC-3′ R: 5′-CACCTTATGGTGTATGAGCGG-3′ |
| GAPDH | F: 5′-GTCTTCACTACCATGGAGAAGG-3′ R: 5′-TCATGGATGACCTTGGCCAG-3′ |
| Control | HFD | |
|---|---|---|
| Fertility (%) | 100 (30/30) | 73.33 (22/30) * |
| Litter size (n = 15) | 8.56 ± 0.72 | 6.54 ± 1.21 * |
| Litter weight (g) (n = 15) | 9.14 ± 1.38 | 8.01 ± 1.14 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fofana, M.; Li, Z.; Li, H.; Li, W.; Wu, L.; Lu, L.; Liu, Q. Decreased Ubiquitination and Acetylation of Histones 3 and 4 Are Associated with Obesity-Induced Disorders of Spermatogenesis in Mice. Toxics 2024, 12, 296. https://doi.org/10.3390/toxics12040296
Fofana M, Li Z, Li H, Li W, Wu L, Lu L, Liu Q. Decreased Ubiquitination and Acetylation of Histones 3 and 4 Are Associated with Obesity-Induced Disorders of Spermatogenesis in Mice. Toxics. 2024; 12(4):296. https://doi.org/10.3390/toxics12040296
Chicago/Turabian StyleFofana, Mahamadou, Zhenyang Li, Han Li, Wenqi Li, Lu Wu, Lu Lu, and Qizhan Liu. 2024. "Decreased Ubiquitination and Acetylation of Histones 3 and 4 Are Associated with Obesity-Induced Disorders of Spermatogenesis in Mice" Toxics 12, no. 4: 296. https://doi.org/10.3390/toxics12040296
APA StyleFofana, M., Li, Z., Li, H., Li, W., Wu, L., Lu, L., & Liu, Q. (2024). Decreased Ubiquitination and Acetylation of Histones 3 and 4 Are Associated with Obesity-Induced Disorders of Spermatogenesis in Mice. Toxics, 12(4), 296. https://doi.org/10.3390/toxics12040296