Exposure to Sub-Lethal Doses of Permethrin Is Associated with Neurotoxicity: Changes in Bioenergetics, Redox Markers, Neuroinflammation and Morphology
Abstract
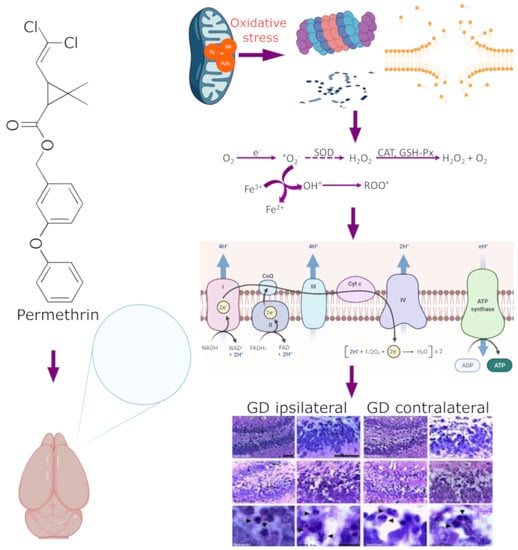
:1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Experimental Groups
2.3. Permethrin Oral Exposure
2.4. Tissue Homogenization
2.5. Total Protein Quantitation by the Lowry Method
2.6. Oxidative Stress Marker Detection
2.7. Protein Carbonyl Assay
2.8. Mitochondrial Isolation
2.9. Mitochondrial O2 Consumption
2.10. RT-PCR Expression for Pro-Inflammatory Cytokines
2.11. Hippocampal Histopathological Analysis
2.12. Statistical Analysis
3. Results
3.1. Permethrin Exposure Induces Lipoperoxidation and Carbonylation
3.2. Antioxidant Marker Determination
3.3. Permethrin Stimulates Cytokine Gene Expression
3.4. Histopathological Analysis
3.5. Permethrin Exposure Alters Mitochondrial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, F.; Souders, C.L., II; Perez-Rodriguez, V.; Martyniuk, C.J. Elucidating Conserved Transcriptional Networks Underlying Pesticide Exposure and Parkinson’s Disease: A Focus on Chemicals of Epidemiological Relevance. Front. Genet. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Hanke, W.; Jurewicz, J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: An overview of current epidemiological evidence. Int. J. Occup. Med. Environ. Health 2004, 17, 223–243. [Google Scholar]
- Valcke, M.; Levasseur, M.E.; Soares da Silva, A.; Wesseling, C. Pesticide exposures and chronic kidney disease of unknown etiology: An epidemiologic review. Environ. Health 2017, 16, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudri, B.S.; Charabi, Y.; Baawain, M.; Ahmed, M. Effects of Pollution on Freshwater Organisms. Water Environ. Res. 2017, 89, 1676–1703. [Google Scholar] [CrossRef]
- Del Prado-Lu, J.L. Insecticide residues in soil, water, and eggplant fruits and farmers’ health effects due to exposure to pesticides. Environ. Health Prev. Med. 2015, 20, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.L. Analysis of Trends of the Types of Pesticide Used, Residues and Related Factors among Farmers in the Largest Vegetable Producing Area in the Philippines. J. Rural Med. 2010, 5, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirsaheb, M.; Limoee, M.; Namdari, F.; Khamutian, R. Organochlorine pesticides residue in breast milk: A systematic review. Med. J. Islam. Repub. Iran. 2015, 29, 228. [Google Scholar]
- Williams, M.K.; Rundle, A.; Holmes, D.; Reyes, M.; Hoepner, L.A.; Barr, D.B.; Camann, D.E.; Perera, F.P.; Whyatt, R.M. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000-2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect. 2008, 116, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, C.B.; Holden, L.; Sturgess, N.; Weiner, M.; Sheets, L.; Sargent, D.; Soderlund, D.M.; Choi, J.S.; Symington, S.; Clark, J.M.; et al. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 2009, 30 (Suppl. 1), S17–S31. [Google Scholar] [CrossRef]
- Chrustek, A.; Holynska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wroblewski, M.; Cwynar, A.; Olszewska-Slonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [Green Version]
- Horton, M.K.; Jacobson, J.B.; McKelvey, W.; Holmes, D.; Fincher, B.; Quantano, A.; Diaz, B.P.; Shabbazz, F.; Shepard, P.; Rundle, A.; et al. Characterization of residential pest control products used in inner city communities in New York City. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 291–301. [Google Scholar] [CrossRef]
- Power, L.E.; Sudakin, D.L. Pyrethrin and pyrethroid exposures in the United States: A longitudinal analysis of incidents reported to poison centers. J. Med. Toxicol. 2007, 3, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol. Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef]
- Soderlund, D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2012, 86, 165–181. [Google Scholar] [CrossRef] [Green Version]
- Gunning, K.; Kiraly, B.; Pippitt, K. Lice and Scabies: Treatment Update. Am. Fam. Phys. 2019, 99, 635–642. [Google Scholar]
- Gunning, K.; Pippitt, K.; Kiraly, B.; Sayler, M. Pediculosis and scabies: Treatment update. Am. Fam. Phys. 2012, 86, 535–541. [Google Scholar]
- Londono-Renteria, B.; Patel, J.C.; Vaughn, M.; Funkhauser, S.; Ponnusamy, L.; Grippin, C.; Jameson, S.B.; Apperson, C.; Mores, C.N.; Wesson, D.M.; et al. Long-Lasting Permethrin-Impregnated Clothing Protects Against Mosquito Bites in Outdoor Workers. Am. J. Trop. Med. Hyg. 2015, 93, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salavastru, C.M.; Chosidow, O.; Boffa, M.J.; Janier, M.; Tiplica, G.S. European guideline for the management of scabies. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1248–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.M.; Poffley, A.; Funkhouser, S.; Driver, J.; Ross, J.; Ospina, M.; Calafat, A.M.; Beard, C.B.; White, A.; Balanay, J.A.; et al. Bioabsorption and effectiveness of long-lasting permethrin-treated uniforms over three months among North Carolina outdoor workers. Parasit Vectors 2019, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Holynska-Iwan, I.; Szewczyk-Golec, K. Pyrethroids: How They Affect Human and Animal Health? Medicina 2020, 56, 582. [Google Scholar] [CrossRef] [PubMed]
- Joshi, U.; Pearson, A.; Evans, J.E.; Langlois, H.; Saltiel, N.; Ojo, J.; Klimas, N.; Sullivan, K.; Keegan, A.P.; Oberlin, S.; et al. A permethrin metabolite is associated with adaptive immune responses in Gulf War Illness. Brain Behav. Immun. 2019, 81, 545–559. [Google Scholar] [CrossRef]
- Shearer, J.J.; Beane Freeman, L.E.; Liu, D.; Andreotti, G.; Hamilton, J.; Happel, J.; Lynch, C.F.; Alavanja, M.C.; Hofmann, J.N. Longitudinal investigation of haematological alterations among permethrin-exposed pesticide applicators in the Biomarkers of Exposure and Effect in Agriculture study. Occup. Environ. Med. 2019, 76, 467–470. [Google Scholar] [CrossRef]
- Sundaramoorthy, R.; Velusamy, Y.; Balaji, A.P.; Mukherjee, A.; Chandrasekaran, N. Comparative cytotoxic and genotoxic effects of permethrin and its nanometric form on human erythrocytes and lymphocytes in vitro. Chem. Biol. Interact. 2016, 257, 119–124. [Google Scholar] [CrossRef]
- Wang, X.; Martinez, M.A.; Dai, M.; Chen, D.; Ares, I.; Romero, A.; Castellano, V.; Martinez, M.; Rodriguez, J.L.; Martinez-Larranaga, M.R.; et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ. Res. 2016, 149, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.M.; Gordon, H.E.; Ludwig, H.D.; Wagner, J.J.; Harn, D.A.; Norberg, T.; Filipov, N.M. Neurochemical and neuroinflammatory perturbations in two Gulf War Illness models: Modulation by the immunotherapeutic LNFPIII. Neurotoxicology 2020, 77, 40–50. [Google Scholar] [CrossRef]
- Kodali, M.; Hattiangady, B.; Shetty, G.A.; Bates, A.; Shuai, B.; Shetty, A.K. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018, 69, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Parihar, V.K.; Hattiangady, B.; Shuai, B.; Shetty, A.K. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology 2013, 38, 2348–2362. [Google Scholar] [CrossRef]
- Parkitny, L.; Middleton, S.; Baker, K.; Younger, J. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunol. 2015, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- Shetty, G.A.; Hattiangady, B.; Upadhya, D.; Bates, A.; Attaluri, S.; Shuai, B.; Kodali, M.; Shetty, A.K. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front. Mol. Neurosci. 2017, 10, 182. [Google Scholar] [CrossRef]
- Nunes, M.E.M.; Schimith, L.E.; da Costa-Silva, D.G.; Lopes, A.R.; Leandro, L.P.; Martins, I.K.; de Mello, R.S.; Hartmann, D.D.; de Carvalho, N.R.; da Rosa, P.C.; et al. Acute Exposure to Permethrin Modulates Behavioral Functions, Redox, and Bioenergetics Parameters and Induces DNA Damage and Cell Death in Larval Zebrafish. Oxid. Med. Cell. Longev. 2019, 2019, 9149203. [Google Scholar] [CrossRef]
- Cantalamessa, F. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Arch. Toxicol. 1993, 67, 510–513. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Camacho-Carranza, R.; Cardenas-Rodriguez, N.; Huerta-Gertrudis, B.; Medina-Campos, O.N.; Mendoza-Cruz, M.; Delgado-Lamas, G.; Espinosa-Aguirre, J.J. Antioxidant activity of Heterotheca inuloides extracts and of some of its metabolites. Toxicology 2010, 276, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Huerta-Gertrudis, B.; Garcia-Cruz, M.E.; Montesinos-Correa, H.; Sanchez-Gonzalez, D.J.; Camacho-Carranza, R.; Espinosa-Aguirre, J.J. Acetonic and Methanolic Extracts of Heterotheca inuloides, and Quercetin, Decrease CCl(4)-Oxidative Stress in Several Rat Tissues. Evid.-Based Complement. Alternat. Med. 2013, 2013, 659165. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Aparicio, L.; Cardenas-Rodriguez, N.; Delgado-Lamas, G.; Pedraza-Chaverri, J.; Montesinos-Correa, H.; Rivera-Espinosa, L.; Torres-Espindola, L.M.; Hernandez, M.E.; Lopez-Aceves, T.; Perez-Lozano, D.L.; et al. Dose-Dependent Behavioral and Antioxidant Effects of Quercetin and Methanolic and Acetonic Extracts from Heterotheca inuloides on Several Rat Tissues following Kainic Acid-Induced Status Epilepticus. Oxid. Med. Cell. Longev. 2019, 2019, 5287507. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Morales, P.; Leon-Contreras, J.C.; Aparicio-Trejo, O.E.; Reyes-Ocampo, J.G.; Medina-Campos, O.N.; Jimenez-Osorio, A.S.; Gonzalez-Reyes, S.; Marquina-Castillo, B.; Hernandez-Pando, R.; Barrera-Oviedo, D.; et al. Fasting reduces oxidative stress, mitochondrial dysfunction and fibrosis induced by renal ischemia-reperfusion injury. Free Radic. Biol. Med. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Reyes-Fermin, L.M.; Briones-Herrera, A.; Tapia, E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic. Biol. Med. 2019, 130, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Bozolla, J.J.; Russell, L.D. (Eds.) Specimen preparation for transmission electron microscopy. In Electron Microscopy.Principles and Techniques for Biologists, 2nd ed.; Jones and Bartlett Publishers, Inc.: Burlington, MA, USA, 1999. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. (Eds.) Neuropathological histotechnology. In Laboratory Methods in Histotechnology; Armed Forces Institute of Pathology: Washington, DC, USA, 1992; pp. 94–95. [Google Scholar]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Cellular responses to stress and toxic insults: Adaptation, injury, and death. In Robbins and Cotran: Pathological Basis of Diseases, 8th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 3–42. [Google Scholar]
- Bordoni, L.; Nasuti, C.; Fedeli, D.; Galeazzi, R.; Laudadio, E.; Massaccesi, L.; Lopez-Rodas, G.; Gabbianelli, R. Early impairment of epigenetic pattern in neurodegeneration: Additional mechanisms behind pyrethroid toxicity. Exp. Gerontol. 2019, 124, 110629. [Google Scholar] [CrossRef]
- Meyer, D.A.; Carter, J.M.; Johnstone, A.F.; Shafer, T.J. Pyrethroid modulation of spontaneous neuronal excitability and neurotransmission in hippocampal neurons in culture. Neurotoxicology 2008, 29, 213–225. [Google Scholar] [CrossRef]
- Carloni, M.; Nasuti, C.; Fedeli, D.; Montani, M.; Amici, A.; Vadhana, M.S.; Gabbianelli, R. The impact of early life permethrin exposure on development of neurodegeneration in adulthood. Exp. Gerontol. 2012, 47, 60–66. [Google Scholar] [CrossRef]
- Fedeli, D.; Carloni, M.; Nasuti, C.; Gambini, A.; Scocco, V.; Gabbianelli, R. Early life permethrin exposure leads to hypervitaminosis D, nitric oxide and catecholamines impairment. Pestic. Biochem. Physiol. 2013, 107, 93–97. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Falcioni, M.L.; Nasuti, C.; Cantalamessa, F.; Imada, I.; Inoue, M. Effect of permethrin insecticide on rat polymorphonuclear neutrophils. Chem. Biol. Interact. 2009, 182, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Carloni, M.; Fedeli, D.; Di Stefano, A.; Marinelli, L.; Cerasa, L.S.; Meda, C.; Maggi, A.; Gabbianelli, R. Effect of 17beta-estradiol on striatal dopaminergic transmission induced by permethrin in early childhood rats. Chemosphere 2014, 112, 496–502. [Google Scholar] [CrossRef]
- Falcioni, M.L.; Nasuti, C.; Bergamini, C.; Fato, R.; Lenaz, G.; Gabbianelli, R. The primary role of glutathione against nuclear DNA damage of striatum induced by permethrin in rats. Neuroscience 2010, 168, 2–10. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.; Tallman, K.A.; Porter, N.A.; Brash, A.R. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001, 276, 20831–20838. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Nasuti, C.; Gabbianelli, R.; Falcioni, M.L.; Di Stefano, A.; Sozio, P.; Cantalamessa, F. Dopaminergic system modulation, behavioral changes, and oxidative stress after neonatal administration of pyrethroids. Toxicology 2007, 229, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Rosita, G.; Manuel, C.; Franco, M.; Cinzia, N.; Donatella, F.; Emiliano, L.; Luca, M.; Roberta, G. Permethrin and its metabolites affect Cu/Zn superoxide conformation: Fluorescence and in silico evidences. Mol. Biosyst. 2015, 11, 208–217. [Google Scholar] [CrossRef]
- Omotoso, G.; Oloyede, O.; Lawal, S.; Gbadamosi, I.; Mutholib, N.; Abdulsalam, F.; Bature, A.; Babalola, A.; Ayeni, B.; Amedu, N. Permethrin exposure affects neurobehavior and cellular characterization in rats’ brain. Environ. Anal. Health Toxicol. 2020, 35, e2020022. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of microglia in CNS inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [Green Version]
- Fogal, B.; Hewett, S.J. Interleukin-1beta: A bridge between inflammation and excitotoxicity? J. Neurochem. 2008, 106, 1–23. [Google Scholar] [CrossRef]
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G.; et al. Tumor Necrosis Factor and Interleukin-1beta Modulate Synaptic Plasticity during Neuroinflammation. Neural Plast. 2018, 2018, 8430123. [Google Scholar] [CrossRef] [Green Version]
- Sierra, A.; Beccari, S.; Diaz-Aparicio, I.; Encinas, J.M.; Comeau, S.; Tremblay, M.E. Surveillance, phagocytosis, and inflammation: How never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014, 2014, 610343. [Google Scholar] [CrossRef] [PubMed]
- Widera, D.; Mikenberg, I.; Elvers, M.; Kaltschmidt, C.; Kaltschmidt, B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Bernardino, L.; Xapelli, S.; Silva, A.P.; Jakobsen, B.; Poulsen, F.R.; Oliveira, C.R.; Vezzani, A.; Malva, J.O.; Zimmer, J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J. Neurosci. 2005, 25, 6734–6744. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, H.; Cao, L.; Li, K.; Huang, Q. Interleukin-1beta inhibits the differentiation of hippocampal neural precursor cells into serotonergic neurons. Brain Res. 2013, 1490, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Neta, G.; Goldman, L.R.; Barr, D.; Apelberg, B.J.; Witter, F.R.; Halden, R.U. Fetal exposure to chlordane and permethrin mixtures in relation to inflammatory cytokines and birth outcomes. Environ. Sci. Technol. 2011, 45, 1680–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallieres, L.; Campbell, I.L.; Gage, F.H.; Sawchenko, P.E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 2002, 22, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Gruol, D.L. IL-6 regulation of synaptic function in the CNS. Neuropharmacology 2015, 96, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Trapero, I.; Cauli, O. Interleukin 6 and cognitive dysfunction. Metab. Brain Dis. 2014, 29, 593–608. [Google Scholar] [CrossRef]
- Ben-Hur, T.; Ben-Menachem, O.; Furer, V.; Einstein, O.; Mizrachi-Kol, R.; Grigoriadis, N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol. Cell. Neurosci. 2003, 24, 623–631. [Google Scholar] [CrossRef]
- Iosif, R.E.; Ahlenius, H.; Ekdahl, C.T.; Darsalia, V.; Thored, P.; Jovinge, S.; Kokaia, Z.; Lindvall, O. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J. Cereb. Blood Flow Metab. 2008, 28, 1574–1587. [Google Scholar] [CrossRef]
- Wu, J.P.; Kuo, J.S.; Liu, Y.L.; Tzeng, S.F. Tumor necrosis factor-alpha modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci. Lett. 2000, 292, 203–206. [Google Scholar] [CrossRef]
- Hossain, M.M.; Liu, J.; Richardson, J.R. Pyrethroid Insecticides Directly Activate Microglia Through Interaction With Voltage-Gated Sodium Channels. Toxicol. Sci. 2017, 155, 112–123. [Google Scholar] [CrossRef]
- Olmos, G.; Llado, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef]
- Meyer, D.A.; Shafer, T.J. Permethrin, but not deltamethrin, increases spontaneous glutamate release from hippocampal neurons in culture. Neurotoxicology 2006, 27, 594–603. [Google Scholar] [CrossRef]
- Baek, B.S.; Kwon, H.J.; Lee, K.H.; Yoo, M.A.; Kim, K.W.; Ikeno, Y.; Yu, B.P.; Chung, H.Y. Regional difference of ROS generation, lipid peroxidation, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch. Pharm. Res. 1999, 22, 361–366. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.; Shetty, A.K.; Abou-Donia, M.B. Subchronic dermal application of N,N-diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Exp. Neurol. 2001, 172, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Jin, X.; Willmore, W.G. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox. Biol. 2014, 2, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.F.; Liu, H.; Ni, H.F.; Lv, L.L.; Zhang, M.H.; Zhang, A.H.; Tang, R.N.; Chen, P.S.; Liu, B.C. Improved mitochondrial function underlies the protective effect of pirfenidone against tubulointerstitial fibrosis in 5/6 nephrectomized rats. PLoS ONE 2013, 8, e83593. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Gassner, B.; Wuthrich, A.; Scholtysik, G.; Solioz, M. The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J. Pharmacol. Exp. Ther. 1997, 281, 855–860. [Google Scholar]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]








| Gene Name | GenBank Accession No. | Oligonucleotide (pb) | Tm (°C) | Forward | Reverse |
|---|---|---|---|---|---|
| IL-1β | NM_031512.2 | 228 | 60 | GATGTTCCCATTAGACAGCTGCAC | GTCTTTCATCACACAGGACAGG |
| IL-6 | NM_012589.1 | 221 | 54 | TGGAGTTCCGTTTCTACCTGGAGT | TGGATGGTCTTGGTCCTTAGCCA |
| TNF-α | HQ201305.1 | 152 | 60 | CTGGCCAATGGCATGGATCTCAAA | TGGTATGAAATGGCAAACCGGCTG |
| beta-actin | AF541940.1 | 217 | 60 | CTGACAGGATGCAGAAGGAGAT | AGTAACAGTCCGCCTAGAAGCA |
| Groups | Cerebellum | Cerebral Cortex | Cerebral Hemispheres | Medulla |
|---|---|---|---|---|
| Sham | 60.90 ± 1.89 | 69.53 ± 5.02 | 50.3 ± 3.28 | 40.63 ± 3.81 |
| Corn oil (CO) | 69.83 ± 5.11 ns | 72.53 ± 4.72 ns | 51.24 ± 4.7 ns | 48.34 ± 3.76 ns |
| PERM 150 | 81.25 ± 5.43 ** | 85.45 ± 6.56 * | 85.45 ± 6.56 ** | 64.90 ± 7.77 ** |
| PERM 300 | 87.80 ± 4.88 ** | 97.77 ± 8.49 ** | 97.77 ± 8.49 *** | 69.95 ± 4.06 *** |
| Treatment | GPx (U/mg/Prot) | GR (U/mg/Prot) | CAT (U/mg/Prot) | SOD (U/mg/Prot) | GST (µmol/CDNBmin/Prot) |
|---|---|---|---|---|---|
| Cerebellum | |||||
| Sham | 0.019 ± 0.001 | 0.013 ± 0.001 | 8.90 ± 0.36 | 2.11 ± 0.171 | 0.036 ± 0.006 |
| Corn oil | 0.020 ± 0.002 ns | 0.014 ± 0.002 ns | 11.24 ± 0.81 ns | 3.06 ± 0.226 * | 0.039 ± 0.016 ns |
| PERM 150 mg/kg | 0.026 ± 0.003 * | 0.019 ± 0.003 * | 13.96 ± 0.74 * | 3.88 ± 0.594 ** | 0.054 ± 0.005 ns |
| PERM 300 mg/kg | 0.029 ± 0.004 ** | 0.021 ± 0.002 ** | 15.48 ± 1.05 ** | 3.92 ± 0.367 *** | 0.068 ± 0.005 ** |
| Prefrontal cortex | |||||
| Sham | 0.012 ± 0.006 | 0.015± 0.006 | 13.50 ± 0.71 | 1.19 ± 0.052 | 0.021 ± 0.001 |
| Corn oil | 0.015 ± 0.001 ns | 0.010 ± 0.001 ns | 14.03 ± 0.88 ns | 1.23 ± 0.14 ns | 0.027 ± 0.003 ns |
| PERM 150 mg/kg | 0.017 ± 0.009 ** | 0.015 ± 0.001 ns | 17.98 ± 1.30 ** | 1.86 ± 0.14 *** | 0.032 ± 0.004 ** |
| PERM 300 mg/kg | 0.016 ± 0.001 ns | 0.014 ± 0.002 ns | 16.66 ± 1.34 * | 1.73 ± 0.14 ** | 0.033 ± 0.004 ** |
| Cerebral hemispheres | |||||
| Sham | 0.012 ± 0.001 | 0.010 ± 0.000 | 7.24 ± 0.06 | 0.86 ± 0.005 | 0.020 ± 0.003 |
| Corn oil | 0.013 ± 0.001 ns | 0.010 ± 0.001 ns | 7.75 ± 0.65 ns | 0.97 ± 0.144 * | 0.022 ± 0.002 ns |
| PERM 150 mg/kg | 0.015 ± 0.002 * | 0.010 ± 0.001 ns | 10.80 ± 0.84 * | 1.09 ± 0.045 ** | 0.029 ± 0.001 ns |
| PERM 300 mg/kg | 0.016 ± 0.001 ** | 0.010 ± 0.001 ns | 10.76 ± 0.73 * | 1.12 ± 0.0640 * | 0.029 ± 0.002 ns |
| Medulla | |||||
| Sham | 0.022 ± 0.000 | 0.062 ± 0.003 | 9.97 ± 0.75 | 3.78 ± 0.66 | 0.021 ± 0.001 |
| Corn oil | 0.021 ± 0.001 ns | 0.070 ± 0.001 ns | 11.07 ± 0.90 ns | 4.98 ± 0.98 ns | 0.018 ± 0.004 ns |
| PERM 150 mg/kg | 0.025 ± 0.002 ns | 0.076 ± 0.00 * | 12.39 ± 0.91 * | 6.72 ± 1.4 * | 0.020 ± 0.003 ns |
| PER 300 mg/kg | 0.025 ± 0.002 ns | 0.080 ± 0.001 ** | 12.88 ± 0.99 ** | 7.7 ± 1.00 ** | 0.018 ± 0.003 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Aceves, T.G.; Coballase-Urrutia, E.; Estrada-Rojo, F.; Vanoye-Carlo, A.; Carmona-Aparicio, L.; Hernández, M.E.; Pedraza-Chaverri, J.; Navarro, L.; Aparicio-Trejo, O.E.; Pérez-Torres, A.; et al. Exposure to Sub-Lethal Doses of Permethrin Is Associated with Neurotoxicity: Changes in Bioenergetics, Redox Markers, Neuroinflammation and Morphology. Toxics 2021, 9, 337. https://doi.org/10.3390/toxics9120337
López-Aceves TG, Coballase-Urrutia E, Estrada-Rojo F, Vanoye-Carlo A, Carmona-Aparicio L, Hernández ME, Pedraza-Chaverri J, Navarro L, Aparicio-Trejo OE, Pérez-Torres A, et al. Exposure to Sub-Lethal Doses of Permethrin Is Associated with Neurotoxicity: Changes in Bioenergetics, Redox Markers, Neuroinflammation and Morphology. Toxics. 2021; 9(12):337. https://doi.org/10.3390/toxics9120337
Chicago/Turabian StyleLópez-Aceves, Teresita Guadalupe, Elvia Coballase-Urrutia, Francisco Estrada-Rojo, América Vanoye-Carlo, Liliana Carmona-Aparicio, María Eugenia Hernández, José Pedraza-Chaverri, Luz Navarro, Omar E. Aparicio-Trejo, Armando Pérez-Torres, and et al. 2021. "Exposure to Sub-Lethal Doses of Permethrin Is Associated with Neurotoxicity: Changes in Bioenergetics, Redox Markers, Neuroinflammation and Morphology" Toxics 9, no. 12: 337. https://doi.org/10.3390/toxics9120337
APA StyleLópez-Aceves, T. G., Coballase-Urrutia, E., Estrada-Rojo, F., Vanoye-Carlo, A., Carmona-Aparicio, L., Hernández, M. E., Pedraza-Chaverri, J., Navarro, L., Aparicio-Trejo, O. E., Pérez-Torres, A., Medina-Campos, O. N., Martínez-Fong, D., Sánchez-Valle, V., Cárdenas-Rodríguez, N., Granados-Rojas, L., Pulido-Camarillo, E., Rodríguez-Mata, V., & León-Sicairos, C. d. R. (2021). Exposure to Sub-Lethal Doses of Permethrin Is Associated with Neurotoxicity: Changes in Bioenergetics, Redox Markers, Neuroinflammation and Morphology. Toxics, 9(12), 337. https://doi.org/10.3390/toxics9120337