Protracted Impairment of Maternal Metabolic Health in Mouse Dams Following Pregnancy Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals, a Pilot Study
Abstract
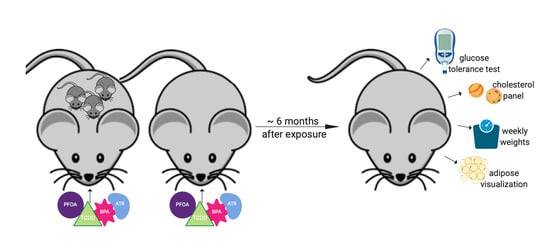
:1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Glucose Tolerance Test
2.3. Serum Lipids
2.4. Visceral Adipose Visualization
2.5. Serum Adipokines
2.6. Serum Corticosterone
2.7. Statistical Analyses
3. Results
3.1. Glucose Tolerance Test
3.2. Serum Lipids
3.3. Visceral Adipose Visualization
3.4. Serum Adipokines
3.5. Serum Corticosterone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catanese, M.C.; Suvorov, A.; Vandenberg, L.N. Beyond a means of exposure: A new view of the mother in toxicology research. Toxicol. Res. 2015, 4, 592–612. [Google Scholar] [CrossRef]
- Rahim, M.N.; Williamson, C.; Kametas, N.A.; Heneghan, M.A. Using pregnancy to assess risk and predict women’s health. EClinicalMedicine 2020, 20. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Butler, A.; Cao-Minh, L.; Galasso, R.; Rizza, R.; Corradin, A.; Cobelli, C.; Butler, P. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010, 53, 2167–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-C.; Fisher, M.; Davis, K.; Arbuckle, T.E.; Sinha, S.K. Identification of chemical mixtures to which Canadian pregnant women are exposed: The MIREC Study. Environ. Int. 2017, 99, 321–330. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Cushing, L.J.; Jesdale, B.M.; Schwartz, J.M.; Guo, W.; Guo, T.; Wang, M.; Harwani, S.; Petropoulou, S.-S.E.; Duong, W. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ. Sci. Technol. 2016, 50, 12464–12472. [Google Scholar] [CrossRef]
- Robinson, O.; Basagaña, X.; Agier, L.; De Castro, M.; Hernandez-Ferrer, C.; Gonzalez, J.R.; Grimalt, J.O.; Nieuwenhuijsen, M.; Sunyer, J.; Slama, R.M. The pregnancy exposome: Multiple environmental exposures in the INMA-Sabadell birth cohort. Environ. Sci. Technol. 2015, 49, 10632–10641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, T.J.; Zota, A.R.; Schwartz, J.M. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 2011, 119, 878–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.-H.; Mínguez-Alarcón, L.; Ford, J.B.; Keller, M.; Seely, E.W.; Messerlian, C.; Petrozza, J.; Williams, P.L.; Ye, X.; Calafat, A.M. Trimester-specific urinary bisphenol A concentrations and blood glucose levels among pregnant women from a fertility clinic. J. Clin. Endocrinol. Metab. 2017, 102, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, R.M.; Ferguson, K.K.; Sheppard, L.; James-Todd, T.; Butts, S.; Chandrasekaran, S.; Swan, S.H.; Barrett, E.S.; Nguyen, R.; Bush, N. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ. Int. 2019, 123, 588–596. [Google Scholar] [CrossRef]
- Vafeiadi, M.; Roumeliotaki, T.; Chalkiadaki, G.; Rantakokko, P.; Kiviranta, H.; Fthenou, E.; Kyrtopoulos, S.A.; Kogevinas, M.; Chatzi, L. Persistent organic pollutants in early pregnancy and risk of gestational diabetes mellitus. Environ. Int. 2017, 98, 89–95. [Google Scholar] [CrossRef] [PubMed]
- James-Todd, T.M.; Chiu, Y.-H.; Messerlian, C.; Mínguez-Alarcón, L.; Ford, J.B.; Keller, M.; Petrozza, J.; Williams, P.L.; Ye, X.; Calafat, A.M. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ. Health 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavia, A.; Chiu, Y.-H.; Brown, F.M.; Mínguez-Alarcón, L.; Ford, J.B.; Keller, M.; Petrozza, J.; Williams, P.L.; Ye, X.; Calafat, A.M. Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environ. Res. 2019, 168, 389–396. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Liu, W.; Li, X.; Hu, J.; Zhang, B.; Xu, S.; Zhou, Y.; Li, J.; Cai, Z. Exposure to bisphenol A substitutes and gestational diabetes mellitus: A prospective cohort study in China. Front. Endocrinol. 2019, 10, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James-Todd, T.M.; Meeker, J.D.; Huang, T.; Hauser, R.; Ferguson, K.K.; Rich-Edwards, J.W.; McElrath, T.F.; Seely, E.W. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int. 2016, 96, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.L.; Zhang, C.; Smarr, M.M.; Lee, S.; Honda, M.; Kannan, K.; Tekola-Ayele, F.; Louis, G.M.B. Persistent organic pollutants and gestational diabetes: A multi-center prospective cohort study of healthy US women. Environ. Int. 2019, 124, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Saldana, T.M.; Basso, O.; Hoppin, J.A.; Baird, D.D.; Knott, C.; Blair, A.; Alavanja, M.C.; Sandler, D.P. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care 2007, 30, 529–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matilla-Santander, N.; Valvi, D.; Lopez-Espinosa, M.-J.; Manzano-Salgado, C.B.; Ballester, F.; Ibarluzea, J.; Santa-Marina, L.; Schettgen, T.; Guxens, M.; Sunyer, J. Exposure to perfluoroalkyl substances and metabolic outcomes in pregnant women: Evidence from the Spanish INMA birth cohorts. Environ. Health Perspect. 2017, 125, 117004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sundaram, R.; Maisog, J.; Calafat, A.M.; Barr, D.B.; Louis, G.M.B. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil. Steril. 2015, 103, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Groth, S.W.; Fernandez, I.D.; Block, R.C.; Thurston, S.W.; Wong, E.; Brunner, J.; Mayo, N.; Kapula, N.; Yu, Y.; Meng, Y. Biological changes in the pregnancy-postpartum period and subsequent cardiometabolic risk—UPSIDE MOMS: A research protocol. Res. Nurs. Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.S.; Groth, S.W.; Preston, E.V.; Kinkade, C.; James-Todd, T. Endocrine-Disrupting Chemical Exposures in Pregnancy: A Sensitive Window for Later-Life Cardiometabolic Health in Women. Curr. Epidemiol. Rep. 2021, 8, 130–142. [Google Scholar] [CrossRef]
- Mitro, S.D.; Sagiv, S.K.; Fleisch, A.F.; Jaacks, L.M.; Williams, P.L.; Rifas-Shiman, S.L.; Calafat, A.M.; Hivert, M.-F.; Oken, E.; James-Todd, T.M. Pregnancy per-and polyfluoroalkyl substance concentrations and postpartum health in Project Viva, a prospective cohort. J. Clin. Endocrinol. Metab. 2020, 105, e3415–e3426. [Google Scholar] [CrossRef]
- Rodríguez-Carmona, Y.; Cantoral, A.; Trejo-Valdivia, B.; Téllez-Rojo, M.M.; Svensson, K.; Peterson, K.E.; Meeker, J.D.; Schnaas, L.; Solano, M.; Watkins, D.J. Phthalate exposure during pregnancy and long-term weight gain in women. Environ. Res. 2019, 169, 26–32. [Google Scholar] [CrossRef]
- Wu, H.; Just, A.C.; Colicino, E.; Calafat, A.M.; Oken, E.; Braun, J.M.; McRae, N.; Cantoral, A.; Pantic, I.; Pizano-Zárate, M.L. The associations of phthalate biomarkers during pregnancy with later glycemia and lipid profiles. Environ. Int. 2021, 155, 106612. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [Green Version]
- Le Magueresse-Battistoni, B.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World J. Biol. Chem. 2017, 8, 108–119. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Rowley, W.R.; Bezold, C.; Arikan, Y.; Byrne, E.; Krohe, S. Diabetes 2030: Insights from yesterday, today, and future trends. Popul. Health Manag. 2017, 20, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.M.; Kalkbrenner, A.E.; Just, A.C.; Yolton, K.; Calafat, A.M.; Sjödin, A.; Hauser, R.; Webster, G.M.; Chen, A.; Lanphear, B.P. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4-and 5-year-old children: The HOME study. Environ. Health Perspect. 2014, 122, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kalloo, G.; Wellenius, G.A.; McCandless, L.; Calafat, A.M.; Sjodin, A.; Karagas, M.; Chen, A.; Yolton, K.; Lanphear, B.P.; Braun, J.M. Profiles and predictors of environmental chemical mixture exposure among pregnant women: The health outcomes and measures of the environment study. Environ. Sci. Technol. 2018, 52, 10104–10113. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Engel, S.M.; Berkowitz, G.S.; Ye, X.; Silva, M.J.; Zhu, C.; Wetmur, J.; Calafat, A.M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.; Padmanabhan, V. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howdeshell, K.L.; Hotchkiss, A.K.; Gray, L.E., Jr. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int. J. Hyg. Environ. Health 2017, 220, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Howdeshell, K.L.; Wilson, V.S.; Furr, J.; Lambright, C.R.; Rider, C.V.; Blystone, C.R.; Hotchkiss, A.K.; Gray, L.E., Jr. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 2008, 105, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, C.V.; Wilson, V.S.; Howdeshell, K.L.; Hotchkiss, A.K.; Furr, J.R.; Lambright, C.R.; Gray, L.E. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol. Pathol. 2009, 37, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Cardon, M.; Medlock-Kakaley, E.; Wilson, V.S.; Gray, L.E., Jr. A mixture of 15 phthalates and pesticides below individual chemical no observed adverse effect levels (NOAELs) produces reproductive tract malformations in the male rat. Environ. Int. 2021, 156, 106615. [Google Scholar] [CrossRef]
- Rajapakse, N.; Silva, E.; Kortenkamp, A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ. Health Perspect. 2002, 110, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortenkamp, A. Low dose mixture effects of endocrine disrupters: Implications for risk assessment and epidemiology. Int. J. Androl. 2008, 31, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [Green Version]
- Cory-Slechta, D.A. Studying toxicants as single chemicals: Does this strategy adequately identify neurotoxic risk? Neurotoxicology 2005, 26, 491–510. [Google Scholar] [CrossRef]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Zheng, F.; Sheng, N.; Guo, X.; Dai, J. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci. Rep. 2015, 5, 11029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, S.; Lambers, D.; Baccarelli, A.; Khoury, J.; Macaluso, M.; Ho, S.-M. Endocrine disruptors: A potential risk factor for gestational diabetes mellitus. Am. J. Perinatol. 2016, 33, 1313–1318. [Google Scholar]
- Ruiz, D.; Becerra, M.; Jagai, J.S.; Ard, K.; Sargis, R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 2018, 41, 193–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Ahn, S.Y.; Song, I.C.; Chung, M.H.; Jang, H.C.; Park, K.S.; Lee, K.-U.; Pak, Y.K.; Lee, H.K. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS ONE 2009, 4, e5186. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, X.; Miao, W.; Wu, T.; Shen, H.; Chen, S.; Li, Y.; Pan, Q.; Fu, Z. Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism. Toxicol. Lett. 2014, 225, 392–400. [Google Scholar] [CrossRef]
- Wei, J.; Lin, Y.; Li, Y.; Ying, C.; Chen, J.; Song, L.; Zhou, Z.; Lv, Z.; Xia, W.; Chen, X. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 2011, 152, 3049–3061. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; García-Arévalo, M.; Quesada, I.; Nadal, Á. Bisphenol-A Treatment during Pregnancy in Mice: A New Window of Susceptibility for the Development of Diabetes in Mothers Later in Life. Endocrinology 2015, 156, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M.S. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 2015, 156, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Moon, M.K.; Jeong, I.-K.; Oh, T.J.; Ahn, H.Y.; Kim, H.H.; Park, Y.J.; Jang, H.C.; Park, K.S. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. 2015, 226, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 2010, 118, 1243–1250. [Google Scholar] [CrossRef] [Green Version]
- Le Magueresse-Battistoni, B.; Multigner, L.; Beausoleil, C.; Rousselle, C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018, 475, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007, 14, 245. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Chen, M.; Wang, J.; Xu, M.; Sun, J.; Ding, L.; Lv, X.; Ma, Q.; Bi, Y.; Liu, R. Bisphenol A promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie diet. Endocrinology 2016, 157, 2333–2345. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Fernandes, A.; Demaegdt, H.; Vandermeiren, K.; Hectors, T.L.; Jorens, P.G.; Blust, R.; Vanparys, C. Evaluation of a screening system for obesogenic compounds: Screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS ONE 2013, 8, e77481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begley, T.; White, K.; Honigfort, P.; Twaroski, M.; Neches, R.; Walker, R. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit. Contam. 2005, 22, 1023–1031. [Google Scholar] [CrossRef]
- Trudel, D.; Horowitz, L.; Wormuth, M.; Scheringer, M.; Cousins, I.T.; Hungerbühler, K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. Int. J. 2008, 28, 251–269. [Google Scholar] [CrossRef]
- Hines, E.P.; White, S.S.; Stanko, J.P.; Gibbs-Flournoy, E.A.; Lau, C.; Fenton, S.E. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol. Cell. Endocrinol. 2009, 304, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Janssen, A.; Staats, M.; Hoogenboom, R.; Kersten, S.; Peijnenburg, A. Perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorononanoic acid (PFNA) increase triglyceride levels and decrease cholesterogenic gene expression in human HepaRG liver cells. Arch. Toxicol. 2020, 94, 3137–3155. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, S.L.; Jones, T.; Herrick, R.L.; Xie, C.; Calafat, A.M.; Pinney, S.M.; Woollett, L.A. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol. Rep. 2016, 3, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, E.S.; Agudelo, J.; Kaye, E.M.; Modaresi, S.M.S.; Pfohl, M.; Bečanová, J.; Wei, W.; Polunas, M.; Goedken, M.; Slitt, A.L. The role of maternal high fat diet on mouse pup metabolic endpoints following perinatal PFAS and PFAS mixture exposure. Toxicology 2021, 462, 152921. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, P.; Spagnolo, A.; Marni, E.; Meazza, L.; Bertollini, R.; Segni, G. Birth defects in the Seveso area after TCDD contamination. JAMA 1988, 259, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.C.; Schnorr, T.M.; Whelan, E.A.; Deddens, J.A.; Dankovic, D.A.; Piacitelli, L.A.; Sweeney, M.H.; Connally, L.B. Paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and birth outcomes of offspring: Birth weight, preterm delivery, and birth defects. Environ. Health Perspect. 2004, 112, 1403–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelli, M.; Piaggi, S.; De Tata, V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced impairment of glucose-stimulated insulin secretion in isolated rat pancreatic islets. Toxicol. Lett. 2005, 156, 307–314. [Google Scholar] [CrossRef]
- Kurita, H.; Yoshioka, W.; Nishimura, N.; Kubota, N.; Kadowaki, T.; Tohyama, C. Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. J. Appl. Toxicol. Int. J. 2009, 29, 689–694. [Google Scholar] [CrossRef]
- Piaggi, S.; Novelli, M.; Martino, L.; Masini, M.; Raggi, C.; Orciuolo, E.; Masiello, P.; Casini, A.; De Tata, V. Cell death and impairment of glucose-stimulated insulin secretion induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the β-cell line INS-1E. Toxicol. Appl. Pharmacol. 2007, 220, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Angrish, M.M.; Dominici, C.Y.; Zacharewski, T.R. TCDD-elicited effects on liver, serum, and adipose lipid composition in C57BL/6 mice. Toxicol. Sci. 2013, 131, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Coban, A.; Filipov, N. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J. Neurochem. 2007, 100, 1177–1187. [Google Scholar] [CrossRef]
- Ross, M.K.; Filipov, N.M. Determination of atrazine and its metabolites in mouse urine and plasma by LC–MS analysis. Anal. Biochem. 2006, 351, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Buoso, E.; di Villa Bianca, R.D.; Paola, R.D.; Morroni, F.; Nocentini, G.; Racchi, M.; Viviani, B.; Corsini, E. Immune and Nervous Systems Interaction in Endocrine Disruptors Toxicity: The Case of Atrazine. Front. Toxicol. 2021, 3, 4. [Google Scholar] [CrossRef]
- Rowe, A.M.; Brundage, K.M.; Barnett, J.B. Developmental immunotoxicity of atrazine in rodents. Basic Clin. Pharmacol. Toxicol. 2008, 102, 139–145. [Google Scholar] [CrossRef]
- Cope, H.A.; Blake, B.E.; Love, C.; McCord, J.; Elmore, S.A.; Harvey, J.B.; Chappell, V.A.; Fenton, S.E. Latent, sex-specific metabolic health effects in CD-1 mouse offspring exposed to PFOA or HFPO-DA (GenX) during gestation. Emerg. Contam. 2021, 7, 219–235. [Google Scholar] [CrossRef]
- Fenton, S.E.; Reiner, J.L.; Nakayama, S.F.; Delinsky, A.D.; Stanko, J.P.; Hines, E.P.; White, S.S.; Lindstrom, A.B.; Strynar, M.J.; Petropoulou, S.-S.E. Analysis of PFOA in dosed CD-1 mice. Part 2: Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009, 27, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Vorderstrasse, B.A.; Cundiff, J.A.; Paige Lawrence, B. A dose-response study of the effects of prenatal and lactational exposure to TCDD on the immune response to influenza a virus. J. Toxicol. Environ. Health Part A 2006, 69, 445–463. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Health Effects Support Document for Perfluorooctanoic Acid (PFOA); US Environmental Protection Agency: Washington, DC, USA, 2016.
- Sobolewski, M.; Conrad, K.; Allen, J.L.; Weston, H.; Martin, K.; Lawrence, B.P.; Cory-Slechta, D.A. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology 2014, 45, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Sobolewski, M.; Allen, J.L.; Morris-Schaffer, K.; Klocke, C.; Conrad, K.; Cory-Slechta, D.A. A novel, ecologically relevant, highly preferred, and non-invasive means of oral substance administration for rodents. Neurotoxicol. Teratol. 2016, 56, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Fields, A.M.; Welle, K.; Ho, E.S.; Mesaros, C.; Susiarjo, M. Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice. Commun. Biol. 2021, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Stillerman, K.P.; Mattison, D.R.; Giudice, L.C.; Woodruff, T.J. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod. Sci. 2008, 15, 631–650. [Google Scholar] [CrossRef]
- Varshavsky, J.; Smith, A.; Wang, A.; Hom, E.; Izano, M.; Huang, H.; Padula, A.; Woodruff, T.J. Heightened Susceptibility: A Review of How Pregnancy and Chemical Exposures Influence Maternal Health. Reprod. Toxicol. 2019, 92, 14–56. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.A. The fetal and maternal consequences of gestational diabetes mellitus. J. Matern.-Fetal Neonatal Med. 2010, 23, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.V.; McHale, C.M.; Webster, T.F.; Lowe, L.; Goodson, W.H., III; La Merrill, M.A.; Rice, G.; Zeise, L.; Zhang, L.; Smith, M.T. Using the Key Characteristics of Carcinogens to Develop Research on Chemical Mixtures and Cancer. Environ. Health Perspect. 2021, 129, 035003. [Google Scholar] [CrossRef]
- Christiansen, S.; Axelstad, M.; Scholze, M.; Johansson, H.K.; Hass, U.; Mandrup, K.; Frandsen, H.L.; Frederiksen, H.; Isling, L.K.; Boberg, J. Grouping of endocrine disrupting chemicals for mixture risk assessment–Evidence from a rat study. Environ. Int. 2020, 142, 105870. [Google Scholar] [CrossRef]
- Kortenkamp, A. Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol. Cell. Endocrinol. 2020, 499, 110581. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Batista, T.M.; Alonso-Magdalena, P.; Vieira, E.; Amaral, M.E.C.; Cederroth, C.R.; Nef, S.; Quesada, I.; Carneiro, E.M.; Nadal, A. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS ONE 2012, 7, e33814. [Google Scholar] [CrossRef]
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 2014, 325, 133–143. [Google Scholar] [CrossRef]
- Zheng, F.; Sheng, N.; Zhang, H.; Yan, S.; Zhang, J.; Wang, J. Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicol. Appl. Pharmacol. 2017, 335, 41–48. [Google Scholar] [CrossRef]
- Reaven, G.M.; Chen, Y.-D.; Jeppesen, J.; Maheux, P.; Krauss, R.M. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J. Clin. Investig. 1993, 92, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B. Lipids, diabetes, and coronary heart disease: Insights from the Framingham Study. Am. Heart J. 1985, 110, 1100–1107. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hartnett, S.; Hanley, A.J.; Rewers, M.J.; Wagenknecht, L.E.; Karter, A.J.; Haffner, S.M. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: The insulin resistance atherosclerosis study. J. Clin. Endocrinol. Metab. 2013, 98, 1622–1630. [Google Scholar] [CrossRef] [Green Version]
- Festa, A.; Williams, K.; Hanley, A.J.; Otvos, J.D.; Goff, D.C.; Wagenknecht, L.E.; Haffner, S.M. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation 2005, 111, 3465–3472. [Google Scholar] [CrossRef] [Green Version]
- Garvey, W.T.; Kwon, S.; Zheng, D.; Shaughnessy, S.; Wallace, P.; Hutto, A.; Pugh, K.; Jenkins, A.J.; Klein, R.L.; Liao, Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003, 52, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brulport, A.; Le Corre, L.; Chagnon, M.-C. Chronic exposure of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology 2017, 390, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Bahcecı, M.; Aydemır, M.; Tuzcu, A. Effects of oral fat and glucose tolerance test on serum lipid profile, apolipoprotein, and CRP concentration, and insulin resistance in patients with polycystic ovary syndrome. Fertil. Steril. 2007, 87, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Somm, E.; Schwitzgebel, V.M.; Toulotte, A.; Cederroth, C.R.; Combescure, C.; Nef, S.; Aubert, M.L.; Hüppi, P.S. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ. Health Perspect. 2009, 117, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Pelloux, V.; Guyot, E.; Tordjman, J.; Bui, L.-C.; Chevallier, A.; Forest, C.; Benelli, C.; Clément, K.; Barouki, R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012, 120, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regnier, S.M.; Sargis, R.M. Adipocytes under assault: Environmental disruption of adipose physiology. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 520–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarmolinsky, J.; Barbieri, N.B.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Schmidt, M.I. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef] [Green Version]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Gui, Y.; Silha, J.V.; Mishra, S.; Murphy, L.J. Changes in adipokine expression during food deprivation in the mouse and the relationship to fasting-induced insulin resistance. Can. J. Physiol. Pharmacol. 2003, 81, 979–985. [Google Scholar] [CrossRef]
- Bertile, F.; Raclot, T. Adipose-derived factors during nutritional transitions. Curr. Nutr. Food Sci. 2006, 2, 127–139. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.M.; Bowles, N.P.; Weil, Z.M.; Pfaff, D.W.; McEwen, B.S. Endocrine and physiological changes in response to chronic corticosterone: A potential model of the metabolic syndrome in mouse. Endocrinology 2010, 151, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Strack, A.M.; Sebastian, R.J.; Schwartz, M.W.; Dallman, M.F. Glucocorticoids and insulin: Reciprocal signals for energy balance. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 268, R142–R149. [Google Scholar] [CrossRef]
- De Boer, S.; Koopmans, S.; Slangen, J.; Van der Gugten, J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: Effect of interstressor interval length. Physiol. Behav. 1990, 47, 1117–1124. [Google Scholar] [CrossRef]
- Baker, M. Statisticians issue warning over misuse of p values. Nat. News 2016, 531, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| EDC | Dose | Solvent | Dose Reference |
|---|---|---|---|
| Atrazine (ATR) | 10 mg/kg | Peanut Oil | Below levels typically shown to cause effects in animal studies [73,74,75,76] |
| Bisphenol-A (BPA) | 50 μg/kg | Peanut Oil | Current oral human daily reference dose (https://www.epa.gov/iris; accessed on 1 September 2021) |
| Perfluorooctanoic acid (PFOA) | 0.10 mg/kg | Distilled water | At or below NOAEL referenced in animal studies in the EPA 2016 document ‘Health Effects Support Document for Perfluorooctanoic Acid (PFOA)’ [80] Below levels typically shown to cause effects in animal studies [65,77] |
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) | 0.036 μg/kg | Anisole diluted in peanut oil | Below levels typically shown to cause effects in animal studies [69,70,72,79] |
| Mixture (MIX) | All the above doses added together | All the above | All the above |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merrill, A.K.; Anderson, T.; Conrad, K.; Marvin, E.; James-Todd, T.; Cory-Slechta, D.A.; Sobolewski, M. Protracted Impairment of Maternal Metabolic Health in Mouse Dams Following Pregnancy Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals, a Pilot Study. Toxics 2021, 9, 346. https://doi.org/10.3390/toxics9120346
Merrill AK, Anderson T, Conrad K, Marvin E, James-Todd T, Cory-Slechta DA, Sobolewski M. Protracted Impairment of Maternal Metabolic Health in Mouse Dams Following Pregnancy Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals, a Pilot Study. Toxics. 2021; 9(12):346. https://doi.org/10.3390/toxics9120346
Chicago/Turabian StyleMerrill, Alyssa K., Timothy Anderson, Katherine Conrad, Elena Marvin, Tamarra James-Todd, Deborah A. Cory-Slechta, and Marissa Sobolewski. 2021. "Protracted Impairment of Maternal Metabolic Health in Mouse Dams Following Pregnancy Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals, a Pilot Study" Toxics 9, no. 12: 346. https://doi.org/10.3390/toxics9120346
APA StyleMerrill, A. K., Anderson, T., Conrad, K., Marvin, E., James-Todd, T., Cory-Slechta, D. A., & Sobolewski, M. (2021). Protracted Impairment of Maternal Metabolic Health in Mouse Dams Following Pregnancy Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals, a Pilot Study. Toxics, 9(12), 346. https://doi.org/10.3390/toxics9120346