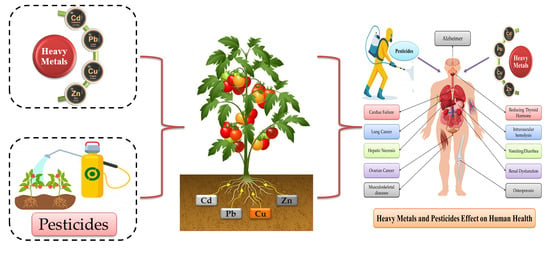
Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications
Abstract
:1. Introduction
2. Sources of Heavy Metals
2.1. Natural Sources of Heavy Metals
2.2. Anthropogenic Sources of Heavy Metals
2.3. Agricultural Sources of Heavy Metals
Fertilizers as a Source of Heavy Metals Accumulation in Agricultural Soil and Plant
3. Pesticides as an Environmental Pollutant
4. Pesticides Classification
4.1. Major Classes of Pesticides
- Classification according to the chemical structure of pesticides
- Classification according to the pest they kill
- Classification according to the mode of entry
4.2. Minor Classes of Pesticides
- Classification according to the toxicity of pesticides
5. Effect of Heavy Metals Toxicity on Agricultural Soil and Plants
5.1. Effect of Heavy Metals Toxicity on Agricultural Soil
5.1.1. Effect of Cadmium Toxicity on Agricultural Soil
5.1.2. Effect of Lead Toxicity on Agricultural Soil
5.1.3. Effect of Copper Toxicity on Agricultural Soil
5.1.4. Effect of Zinc Toxicity on Agricultural Soil
5.2. Effect of Heavy Metals Toxicity on Plants
5.2.1. Effect of Cadmium Toxicity on Plant
5.2.2. Effect of Lead Toxicity on Plant
5.2.3. Effect of Copper Toxicity on Plant
5.2.4. Effect of Zinc Toxicity on Plant
6. Effect of Pesticides Toxicity on Agricultural Soil and Plants
6.1. Effect of Pesticides Toxicity on Agricultural Soil
6.2. Effect of Pesticides Toxicity on Plants
7. Synergism and Antagonism between Heavy Metals and Pesticides in Agricultural Soil and Plant
8. Effect of Heavy Metals and Pesticides Toxicity on Human Health
8.1. Effect of Heavy Metals Toxicity on Human Health
8.1.1. Effect of Heavy Metal Toxicity on Children’s Health
8.1.2. Effect of Heavy Metal Toxicity on Adults
8.2. Effect of Pesticides Toxicity on Human Health
8.3. Combined Toxic Effects of Mixtures of Heavy Metals and Pesticides on Human Health
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bhunia, P. Environmental Toxicants and Hazardous Contaminants: Recent Advances in Technologies for Sustainable Development. J. Hazard. Toxic. Radioact. Waste 2017, 21, 02017001. [Google Scholar] [CrossRef]
- Chin, N.P. Environmental toxins: Physical, social, and emotional. Breastfeed. Med. 2010, 5, 223–224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yan, L.; Liu, J.; Zhang, Z.; Tan, C. Removal of different kinds of heavy metals by novel PPG-nZVI beads and their application in simulated stormwater infiltration facility. Appl. Sci. 2019, 9, 4213. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Critics 2014, 3, 24–38. [Google Scholar]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Cho-Ruk, K.; Kurukote, J.; Supprung, P.; Vetayasuporn, S. Perennial plants in the phytoremediation of lead-contaminated soils. Biotechnology 2006, 5, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 1–31. [Google Scholar] [CrossRef]
- Pastor, S.; Creus, A.; Parrón, T.; Cebulska-Wasilewska, A.; Siffel, C.; Piperakis, S.; Marcos, R. Biomonitoring of four European populations occupationally exposed to pesticides: Use of micronuclei as biomarkers. Mutagenesis 2003, 18, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk-Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: london, UK, 2016. [Google Scholar]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Ni, Z.; Qu, M.; Zhong, D.; Ye, C.; Tang, F. Pesticides in persimmons, jujubes and soil from China: Residue levels, risk assessment and relationship between fruits and soils. Sci. Total Environ. 2016, 542, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Lefrancq, M.; Imfeld, G.; Payraudeau, S.; Millet, M. Kresoxim methyl deposition, drift and runoff in a vineyard catchment. Sci. Total Environ. 2013, 442, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Verger, P.J.P.; Boobis, A.R. Reevaluate pesticides for food security and safety. Science 2013, 341, 717–718. [Google Scholar] [CrossRef]
- Ying, L.; Shaogang, L.; Xiaoyang, C. Assessment of heavy metal pollution and human health risk in urban soils of a coal mining city in East China. Hum. Ecol. Risk Assess. An Int. J. 2016, 22, 1359–1374. [Google Scholar] [CrossRef]
- Tong, S.; Li, H.; Wang, L.; Tudi, M.; Yang, L. Concentration, Spatial Distribution, Contamination Degree and Human Health Risk Assessment of Heavy Metals in Urban Soils across China between 2003 and 2019—A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3099. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Li, L.Y.; Chen, H. Assessment of metals pollution and health risk in dust from nursery schools in Xi’an, China. Environ. Res. 2014, 128, 27–34. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, A. Growing up with pesticides. Science 2013, 341, 740–741. [Google Scholar] [CrossRef]
- Sharon, M.; Bhawana, M.; Anita, S.; Gothecha, V.K. A short review on how pesticides affect human health. Int. J. Ayurvedic Herb. Med. 2012, 2, 935–946. [Google Scholar]
- Wickerham, E.L.; Lozoff, B.; Shao, J.; Kaciroti, N.; Xia, Y.; Meeker, J.D. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ. Int. 2012, 47, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roozbahani, M.M.; Sobhanardakani, S.; Karimi, H.; Sorooshnia, R. Natural and Anthropogenic Source of Heavy Metals Pollution in the Soil Samples of an Industrial Complex; a Case Study. Iran. J. Toxicol. 2015, 9, 1336–1341. [Google Scholar]
- Sutkowska, K.; Teper, L.; Czech, T.; Hulok, T.; Olszak, M.; Zogala, J. Quality of Peri-Urban Soil Developed from Ore-Bearing Carbonates: Heavy Metal Levels and Source Apportionment Assessed Using Pollution Indices. Minerals 2020, 10, 1140. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Bradl, H.B. Sources and origins of heavy metals. In Interface Science and Technology; Bradl, H.B., Ed.; Elsevier B.V.: london, UK, 2005; Volume 6, pp. 1–27. [Google Scholar]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar] [PubMed]
- Cannon, H.L.; Connally, G.G.; Epstein, J.B.; Parker, J.G.; Thornton, I.; Wixson, G. Rocks: Geological sources of most trace elements. In Report to the Workshop at South Scas Plantation Captiva Island, FL, US. Geochem Environ; 1978; Volume 3, pp. 17–31. [Google Scholar]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; IntechOpen: london, UK, 2018; pp. 115–132. [Google Scholar]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Alengebawy, A.; Ai, P.; Jin, K.; Chen, M.; Pan, Y. Techno-Economic Assessment of Three Modes of Large-Scale Crop Residue Utilization Projects in China. Energies 2020, 13, 3729. [Google Scholar] [CrossRef]
- Alengebawy, A.; Jin, K.; Ran, Y.; Peng, J.; Zhang, X.; Ai, P. Advanced pre-treatment of stripped biogas slurry by polyaluminum chloride coagulation and biochar adsorption coupled with ceramic membrane filtration. Chemosphere 2021, 267, 129197. [Google Scholar] [CrossRef]
- Cai, L.M.; Wang, Q.S.; Wen, H.H.; Luo, J.; Wang, S. Heavy metals in agricultural soils from a typical township in Guangdong Province, China: Occurrences and spatial distribution. Ecotoxicol. Environ. Saf. 2019, 168, 184–191. [Google Scholar] [CrossRef]
- Chen, X.X.; Liu, Y.M.; Zhao, Q.Y.; Cao, W.Q.; Chen, X.P.; Zou, C.Q. Health risk assessment associated with heavy metal accumulation in wheat after long-term phosphorus fertilizer application. Environ. Pollut. 2020, 262, 114348. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Naidu, R. Role of Phosphorus in (Im)mobilization and Bioavailability of Heavy Metals in the Soil-Plant System BT—Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Albert, L.A., Bro-Rasmussen, F., Crosby, D.G., de Voogt, P., Frehse, H., Hutzinger, O., Mayer, F.L., Morgan, D.P., Park, D.L., et al., Eds.; Springer: New York, NY, USA, 2003; pp. 1–44. ISBN 978-0-387-21725-3. [Google Scholar]
- Ai, P.; Jin, K.; Alengebawy, A.; Elsayed, M.; Meng, L.; Chen, M.; Ran, Y. Effect of application of different biogas fertilizer on eggplant production: Analysis of fertilizer value and risk assessment. Environ. Technol. Innov. 2020, 19, 101019. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Li, Z.; Teng, Y.; Christie, P.; Luo, Y. Effects of long-term fertilizer applications on peanut yield and quality and plant and soil heavy metal accumulation. Pedosphere 2020, 30, 555–562. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Li, H.; Cheng, F. Evaluating heavy metal accumulation and potential risks in soil-plant systems applied with magnesium slag-based fertilizer. Chemosphere 2018, 197, 382–388. [Google Scholar] [CrossRef]
- Liu, Y.M.; Liu, D.Y.; Zhang, W.; Chen, X.X.; Zhao, Q.Y.; Chen, X.P.; Zou, C.Q. Health risk assessment of heavy metals (Zn, Cu, Cd, Pb, As and Cr) in wheat grain receiving repeated Zn fertilizers. Environ. Pollut. 2020, 257, 113581. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. Pestic. Asp. 2014, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Saravi, S.S.S.; Dehpour, A.R. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci. 2016, 145, 255–264. [Google Scholar] [CrossRef]
- Saravi, S.S.S.; Shokrzadeh, M. Role of pesticides in human life in the modern age: A review. In Pesticides in the Modern World-Risks and Benefits; Stoytcheva, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 3–12. [Google Scholar]
- Zhang, W. Global pesticide use: Profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 2018, 8, 1–27. [Google Scholar]
- Pimentel, D. Pesticides and pest control. In Integrated pest management: Innovation-development process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 83–87. [Google Scholar]
- Zhang, W.-J.; van der Werf, W.; Pang, Y. A simulation model for vegetable-insect pest-insect nucleopolyhedrovirus epidemic system. J. Environ. Entomol. 2011, 33, 283–301. [Google Scholar]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; De, A., Bose, R., Kumar, A., Mozumdar, S., Eds.; Springer: New Delhi, India, 2014; ISBN 978-81-322-1688-9. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Top Pesticide Using Countries. Available online: https://www.worldatlas.com/articles/top-pesticide-consuming-countries-of-the-world.html (accessed on 30 August 2020).
- Abdel Khalek, S.T.; Mostafa, Z.K.; Hassan, H.A.; Abd El-Bar, M.M.; Abu El-Hassan, G.M.M. A New List to the Entomofauna Associated with Faba Bean, Vicia faba L.(Fabales: Fabaceae) Grown in El-Kharga Oasis, New Valley Governorate, Egypt. Egypt. Acad. J. Biol. Sci. 2018, 11, 95–100. [Google Scholar] [CrossRef]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. J. Obstet. Gynecol. Neonatal Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Drum, C. Soil Chemistry of Pesticides; PPG Industries, Inc.: Pittsburgh, PA, USA, 1980. [Google Scholar]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides classification and its impact on environment. Int. J. Curr. Microbiol. Appl. Sci 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Lippmann, M.; Leikauf, G.D. Introduction and background. Environ. Toxicants Hum. Expo. Their Health Eff. 2020, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Yadav, I.C.; Devi, N.L. Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 2017, 6, 140–158. [Google Scholar]
- Fishel, F.M.; Ferrell, J.A. Managing pesticide drift. EDIS 2010, 7, 118806. [Google Scholar]
- Katagi, T. Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2010; pp. 1–132. [Google Scholar]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. 2009. Available online: https://apps.who.int/iris/handle/10665/44188 (accessed on 15 September 2020).
- World Health Organization (WHO). Permissible Limits of Heavy Metals in Soil and Plants; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Osmani, M.; Bani, A.; Hoxha, B. Heavy Metals and Ni Phytoextractionin in the Metallurgical Area Soils in Elbasan. Albanian J. Agric. Sci. 2015, 14, 414–419. [Google Scholar]
- Chrastný, V.; Vaněk, A.; Teper, L.; Cabala, J.; Procházka, J.; Pechar, L.; Drahota, P.; Penížek, V.; Komárek, M.; Novák, M. Geochemical position of Pb, Zn and Cd in soils near the Olkusz mine/smelter, South Poland: Effects of land use, type of contamination and distance from pollution source. Environ. Monit. Assess. 2012, 184, 2517–2536. [Google Scholar] [CrossRef]
- Raţiu, I.-A.; Beldean-Galea, M.S.; Bocoş-Binţinţan, V.; Costea, D.-D. Priority Pollutants Present in the Tisza River Hydrographic Basin and their Effects on Living Organisms. Jordan J. Chem. 2018, 13, 15–33. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy metal contamination in rice-producing soils of Hunan province, China and potential health risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef] [PubMed]
- Chrastný, V.; Čadková, E.; Vaněk, A.; Teper, L.; Cabala, J.; Komárek, M. Cadmium isotope fractionation within the soil profile complicates source identification in relation to Pb-Zn mining and smelting processes. Chem. Geol. 2015, 405, 1–9. [Google Scholar] [CrossRef]
- Liao, M.; Luo, Y.K.; Zhao, X.M.; Huang, C.Y. Toxicity of cadmium to soil microbial biomass and its activity: Effect of incubation time on Cd ecological dose in a paddy soil. J. Zhejiang Univ. Sci. 2005, 6 B, 324–330. [Google Scholar] [CrossRef]
- Raiesi, F.; Sadeghi, E. Interactive effect of salinity and cadmium toxicity on soil microbial properties and enzyme activities. Ecotoxicol. Environ. Saf. 2019, 168, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Oumenskou, H.; El Baghdadi, M.; Barakat, A.; Aquit, M.; Ennaji, W.; Karroum, L.A.; Aadraoui, M. Assessment of the heavy metal contamination using GIS-based approach and pollution indices in agricultural soils from Beni Amir irrigated perimeter, Tadla plain, Morocco. Arab. J. Geosci. 2018, 11, 692. [Google Scholar] [CrossRef]
- An, Y.J. Soil ecotoxicity assessment using cadmium sensitive plants. Environ. Pollut. 2004, 127, 21–26. [Google Scholar] [CrossRef]
- Qi, X.; Xu, X.; Zhong, C.; Jiang, T.; Wei, W.; Song, X. Removal of Cadmium and Lead from Contaminated Soils Using Sophorolipids from Fermentation Culture of Starmerella bombicola CGMCC 1576 Fermentation. Int. J. Environ. Res. Public Health 2018, 15, 2334. [Google Scholar] [CrossRef] [Green Version]
- Dotaniya, M.L.; Dotaniya, C.K.; Solanki, P.; Meena, V.D.; Doutaniya, R.K. Lead Contamination and Its Dynamics in Soil–Plant System. In Lead in Plants and the Environment; Gupta, D.K., Chatterjee, S., Walther, C., Eds.; Springer: Cham, Switzerland, 2020; pp. 83–98. [Google Scholar]
- Lan, M.M.; Liu, C.; Liu, S.J.; Qiu, R.L.; Tang, Y.T. Phytostabilization of cd and pb in highly polluted farmland soils using ramie and amendments. Int. J. Environ. Res. Public Health 2020, 17, 1661. [Google Scholar] [CrossRef] [Green Version]
- Vega, F.A.; Andrade, M.L.; Covelo, E.F. Influence of soil properties on the sorption and retention of cadmium, copper and lead, separately and together, by 20 soil horizons: Comparison of linear regression and tree regression analyses. J. Hazard. Mater. 2010, 174, 522–533. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef] [Green Version]
- Placek, A.; Grobelak, A.; Kacprzak, M. Improving the phytoremediation of heavy metals contaminated soil by use of sewage sludge. Int. J. Phytoremediation 2016, 18, 605–618. [Google Scholar] [CrossRef]
- Khan, S.; Hesham, A.E.L.; Qiao, M.; Rehman, S.; He, J.Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef]
- Vlcek, V.; Pohanka, M. Adsorption of copper in soil and its dependence on physical and chemical properties. Acta Univ. Agric. Silvic. Mendelianae Brun. 2018, 66, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Keiblinger, K.M.; Schneider, M.; Gorfer, M.; Paumann, M.; Deltedesco, E.; Berger, H.; Jöchlinger, L.; Mentler, A.; Zechmeister-Boltenstern, S.; Soja, G.; et al. Assessment of Cu applications in two contrasting soils—effects on soil microbial activity and the fungal community structure. Ecotoxicology 2018, 27, 217–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brun, L.A.; Le Corff, J.; Maillet, J. Effects of elevated soil copper on phenology, growth and reproduction of five ruderal plant species. Environ. Pollut. 2003, 122, 361–368. [Google Scholar] [CrossRef]
- Caetano, A.L.; Marques, C.R.; Gonçalves, F.; da Silva, E.F.; Pereira, R. Copper toxicity in a natural reference soil: Ecotoxicological data for the derivation of preliminary soil screening values. Ecotoxicology 2016, 25, 163–177. [Google Scholar] [CrossRef]
- Cordero, I.; Snell, H.; Bardgett, R.D. High throughput method for measuring urease activity in soil. Soil Biol. Biochem. 2019, 134, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gülser, F.; Erdoǧan, E. The effects of heavy metal pollution on enzyme activities and basal soil respiration of roadside soils. Environ. Monit. Assess. 2008, 145, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, X.; Zhang, W.; Wang, J.; Zhu, L.; Wang, J.; Wei, Z.; Ahmad, Z. Separate and joint eco-toxicological effects of sulfadimidine and copper on soil microbial biomasses and ammoxidation microorganisms abundances. Chemosphere 2019, 228, 556–564. [Google Scholar] [CrossRef]
- Frenk, S.; Ben-Moshe, T.; Dror, I.; Berkowitz, B.; Minz, D. Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS ONE 2013, 8, e84441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, J.L.A.; Ernakovich, J.G.; Judy, J.D.; Farrell, M.; Whatmuff, M.; Kirby, J. Long-term effects of copper exposure to agricultural soil function and microbial community structure at a controlled and experimental field site. Environ. Pollut. 2020, 263, 114411. [Google Scholar] [CrossRef] [PubMed]
- Njinga, R.L.; Moyo, M.N.; Abdulmaliq, S.Y. Analysis of Essential Elements for Plants Growth Using Instrumental Neutron Activation Analysis. Int. J. Agron. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Mertens, J.; Smolders, E. Zinc. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 22, pp. 465–493. [Google Scholar]
- Łukowski, A.; Dec, D. Influence of Zn, Cd, and Cu fractions on enzymatic activity of arable soils. Environ. Monit. Assess. 2018, 190, 278. [Google Scholar] [CrossRef] [Green Version]
- Barman, H.; Das, S.K.; Roy, A. Zinc in Soil Environment for Plant Health and Management Strategy. Univers. J. Agric. Res. 2018, 6, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Pietrzykowski, M.; Antonkiewicz, J.; Gruba, P.; Pajak, M. Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill. Open Chem. 2018, 16, 1143–1152. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Gargiulo, L.; Mele, G. Natural restoration of soils on mine heaps with similar technogenic parent material: A case study of long-term soil evolution in Silesian-Krakow Upland Poland. Geoderma 2016, 261, 141–150. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Effect of cadmium, copper and zinc on plants, soil microorganisms and soil enzymes. J. Elem. 2013, 18, 769–796. [Google Scholar] [CrossRef]
- Cabala, J.; Teper, L. Metalliferous constituents of rhizosphere soils contaminated by Zn-Pb mining in southern Poland. Water. Air. Soil Pollut. 2007, 178, 351–362. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium Toxicity in Plants and Role of Mineral Nutrients in Its Alleviation. Am. J. Plant Sci. 2012, 03, 1476–1489. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Pinelli, E.; Pourrut, B.; Silvestre, J.; Dumat, C. Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol. Environ. Saf. 2011, 74, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Jibril, S.A.; Hassan, S.A.; Ishak, C.F.; Megat Wahab, P.E. Cadmium Toxicity Affects Phytochemicals and Nutrient Elements Composition of Lettuce ( Lactuca sativa L.). Adv. Agric. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Ge, N.; Guo, J.; Zhu, L.; Ma, Z.; Cheng, S.; Wang, J. Enterobacter asburiae Reduces Cadmium Toxicity in Maize Plants by Repressing Iron Uptake-Associated Pathways. J. Agric. Food Chem. 2019, 67, 10126–10136. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, Q.; Wang, X.; Wei, M.; Yang, F.; Xu, H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hortic. (Amsterdam). 2010, 123, 521–530. [Google Scholar] [CrossRef]
- Schützendübel, A.; Nikolova, P.; Rudolf, C.; Polle, A. Cadmium and H2O2-induced oxidative stress in Populus x canescens roots. Plant Physiol. Biochem. 2002, 40, 577–584. [Google Scholar] [CrossRef]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants: From Physiology to Remediation; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 163–183. ISBN 9780128148655. [Google Scholar]
- Seregin, I.V.; Kozhevnikova, A.D. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008, 55, 1–22. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Rossi, M.L.; Martinelli, A.P.; Peres, L.E.P.; Medici, L.O.; Lea, P.J.; Azevedo, R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 2009, 67, 387–394. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. N. Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Uzu, G.; Sobanska, S.; Aliouane, Y.; Pradere, P.; Dumat, C. Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environ. Pollut. 2009, 157, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, P.; Rajguru, A.B.; Dudhe, M.Y.; Mathur, J. Efficacy of lead (Pb) phytoextraction of five varieties of Helianthus annuus L. from contaminated soil. Environ. Technol. Innov. 2020, 18, 100718. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration - A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.; Kanu, A.S.; Deng, Q.; Mo, Z.; Pan, S.; Tian, H.; Tang, X. Lead (Pb) toxicity; physio-biochemical mechanisms, grain yield, quality, and Pb distribution proportions in scented rice. Front. Plant Sci. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—a mini review. Environ. Sci. Pollut. Res. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Gichner, T.; Žnidar, I.; Száková, J. Evaluation of DNA damage and mutagenicity induced by lead in tobacco plants. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 2008, 652, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.M.; Kumar, S.G.; Jyothsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 2005, 60, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nas, F.S. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Ecol. Environ. Sci. 2018, 3, 265–268. [Google Scholar] [CrossRef]
- Cimrin, K.M.; Turan, M.; Kapur, B. Effect of elemental sulphur on heavy metals solubility and remediation by plants in calcareous soils. Fresenius Environ. Bull. 2007, 16, 1113–1120. [Google Scholar]
- Hamid, N.; Bukhari, N.; Jawaid, F. Physiological responses of Phaseolus vulgaris to different lead concentrations. Pakistan J. Bot. 2010, 42, 239–246. [Google Scholar]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Chiou, W.Y.; Hsu, F.C. Copper toxicity and prediction models of copper content in leafy vegetables. Sustainability 2019, 11, 6215. [Google Scholar] [CrossRef] [Green Version]
- Bjuhr, J. Trace metals in soils irrigated with waste water in a periurban area downstream Hanoi City, Vietnam. Semin. Pap. 2007, 1–50. [Google Scholar]
- Kopittke, P.M.; Menzies, N.W.; de Jonge, M.D.; Mckenna, B.A.; Donner, E.; Webb, R.I.; Paterson, D.J.; Howard, D.L.; Ryan, C.G.; Glover, C.J.; et al. In situ distribution and speciation of toxic copper, nickel, and zinc in hydrated roots of cowpea. Plant Physiol. 2011, 156, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.H.; Tabaldi, L.A.; Miyazaki, F.R.; Pilecco, M.; Kassab, S.O.; Bigaton, D. Absorção foliar de cobre por plantas de milho: Efeitos no crescimento e rendimento. Cienc. Rural 2013, 43, 1561–1568. [Google Scholar] [CrossRef]
- Aly, A.A.; Mohamed, A.A. The impact of copper ion on growth, thiol compounds and lipid peroxidation in two maize cultivars (Zea mays L.) grown in vitro. Aust. J. Crop Sci. 2012, 6, 541–549. [Google Scholar]
- Song, C.; Yan, Y.; Rosado, A.; Zhang, Z.; Castellarin, S.D. ABA alleviates uptake and accumulation of zinc in grapevine (Vitis vinifera l.) by inducing expression of ZIP and detoxification-related genes. Front. Plant Sci. 2019, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants: Tansley review. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Hafeez, B. Role of Zinc in Plant Nutrition- A Review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Liang, J.; Yang, W. Effects of Zinc and Copper Stress on Antioxidant System of Olive Leaves. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 52058. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Kochian, L.V. Toxicity of Zinc and Copper to Brassica Species: Implications for Phytoremediation. J. Environ. Qual. 1997, 26, 776–781. [Google Scholar] [CrossRef]
- Hammerschmitt, R.K.; Tiecher, T.L.; Facco, D.B.; Silva, L.O.S.; Schwalbert, R.; Drescher, G.L.; Trentin, E.; Somavilla, L.M.; Kulmann, M.S.S.; Silva, I.C.B.; et al. Copper and zinc distribution and toxicity in ‘Jade’ / ‘Genovesa’ young peach tree. Sci. Hortic. 2020, 259, 108763. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Abidine Triqui, Z.E.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Loi, N.N.; Sanzharova, N.I.; Shchagina, N.I.; Mironova, M.P. The Effect of Cadmium Toxicity on the Development of Lettuce Plants on Contaminated Sod-Podzolic Soil. Russ. Agric. Sci. 2018, 44, 49–52. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Plants. In Trace Elements in Soils and Plants Fourth Edition; Kabata-Pendias, A., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2010; pp. 93–122. ISBN 9781420093704. [Google Scholar]
- Zhou, J.; Zhang, Z.; Zhang, Y.; Wei, Y.; Jiang, Z. Effects of lead stress on the growth, physiology, and cellular structure of privet seedlings. PLoS ONE 2018, 13, e0191139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagos, P.; Ballabio, C.; Lugato, E.; Jones, A.; Borrelli, P.; Scarpa, S.; Orgiazzi, A.; Montanarella, L. Potential Sources of Anthropogenic Copper Inputs to European Agricultural Soils. Sustainability 2018, 10, 2380. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Wang, F.; Xu, C.; Pan, N.; Lin, J.; Zhao, R.; Yang, Y.; Luo, H. Source apportionment of heavy metals in agricultural soil based on PMF: A case study in Hexi Corridor, northwest China. Chemosphere 2018, 193, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, D.; Giannandrea, F.; Gallo, M.; Turci, R.; Cattaruzza, M.S.; Lombardo, F.; Lenzi, A.; Gandini, L. Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J. Endocrinol. Invest. 2015, 38, 745–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO) Agrochemicals, Health and Environment: Directory of Resources. 2017. Available online: https://www.who.int/heli/risks/toxics/chemicalsdirectory/en/ (accessed on 24 October 2020).
- El-Wakeil, N.; Gaafar, N.; Sallam, A.; Volkmar, C. Side Effects of Insecticides on Natural Enemies and Possibility of Their Integration in Plant Protection Strategies. In Insecticides - Development of Safer and More Effective Technologies; Trdan, S., Ed.; IntechOpen: London, UK, 2013; pp. 1–56. [Google Scholar]
- Zaka, S.M.; Iqbal, N.; Saeed, Q.; Akrem, A.; Batool, M.; Khan, A.A.; Anwar, A.; Bibi, M.; Azeem, S.; Rizvi, D.E.N.; et al. Toxic effects of some insecticides, herbicides, and plant essential oils against Tribolium confusum Jacquelin du val (Insecta: Coleoptera: Tenebrionidae). Saudi J. Biol. Sci. 2019, 26, 1767–1771. [Google Scholar] [CrossRef]
- Ligor, M.; Bukowska, M.; Ratiu, I.-A.; Gadzała-Kopciuch, R.; Buszewski, B. Determination of Neonicotinoids in Honey Samples Originated from Poland and Other World Countries. Molecules 2020, 25, 5817. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Responses of plants to pesticide toxicity: An overview. Planta Daninha 2019, 37, e019184291. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R.; Tejada Moral, M. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Biondi, A.; Desneux, N.; Siscaro, G.; Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 2012, 87, 803–812. [Google Scholar] [CrossRef]
- Han, Y.; Mo, R.; Yuan, X.; Zhong, D.; Tang, F.; Ye, C.; Liu, Y. Pesticide residues in nut-planted soils of China and their relationship between nut/soil. Chemosphere 2017, 180, 42–47. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Popescu, R.; Dumitrescu, G.; Ciochina, L.P.; Mituletu, M.; Vlad, D.C. The effect of some insecticides on soil microorganisms based on enzymatic and bacteriological analyses. Rom. Biotechnol. Lett. 2015, 20, 10439–10447. [Google Scholar]
- AL-Ani, M.A.M.; Hmoshi, R.M.; Kanaan, I.A.; Thanoon, A.A. Effect of pesticides on soil microorganisms. J. Phys. Conf. Ser. 2019, 1294, 072007. [Google Scholar] [CrossRef]
- Yousaf, S.; Khan, S.; Aslam, M.T. Effect of pesticides on the soil microbial activity. Pak. J. Zool. 2013, 45, 1063–1067. [Google Scholar]
- Goswami, M.R.; Pati, U.K.; Chowdhury, A.; Mukhopadhyay, A. Studies on the effect of cypermethrin on soil microbial biomass and its activity in an alluvial soil. Int. J. Agric. Food Sci. 2013, 3, 1–9. [Google Scholar]
- Haleem, A.M.; Kasim, S.A.; Al-Timimy, J.A. Effect of some organophosphorous insecticides on soil microorganisms populations under lab condition. World Environ. 2013, 3, 170–173. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Hari, K.; Saravanan, V.S.; Sa, T. Influence of pesticides on the growth rate and plant-growth promoting traits of Gluconacetobacter diazotrophicus. Pestic. Biochem. Physiol. 2006, 84, 143–154. [Google Scholar] [CrossRef]
- Sannino, F.; Gianfreda, L. Pesticide influence on soil enzymatic activities. Chemosphere 2001, 45, 417–425. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, D.K. Total bacterial and fungal population after chlorpyrifos and quinalphos treatments in groundnut (Arachis hypogaea L.) soil. Chemosphere 2004, 55, 197–205. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Sawicka, A. Effect of Carbendazim, Imazetapir and Thiram on Nitrogenase Activity, Number of Microorganisms in Soil and Yield of Hybrid Lucerne (Medicago media). Polish J. Environ. Stud. 2002, 11, 737–744. [Google Scholar]
- Madhuri, R.J.; Rangaswamy, V. Influence of selected insecticides on phosphatase activity in groundnut (Arachis hypogeae L.) soils. J. Environ. Biol. 2002, 23, 393–397. [Google Scholar] [PubMed]
- Mayanglambam, T.; Vig, K.; Singh, D.K. Quinalphos persistence and leaching under field conditions and effects of residues on dehydrogenase and alkaline phosphomonoesterases activities in soil. Bull. Environ. Contam. Toxicol. 2005, 75, 1067–1076. [Google Scholar] [CrossRef]
- Milošević, N.A.; Govedarica, M.M. Effect of herbicides on microbiological properties of soil. Zb. Matice Srp. za Prir. Nauk. 2002, 5–21. [Google Scholar] [CrossRef]
- Kremer, R.J.; Means, N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur. J. Agron. 2009, 31, 153–161. [Google Scholar] [CrossRef]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The self-assembly of lignin and its application in nanoparticle synthesis: A short review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar] [CrossRef]
- Arif, M.A.S.; Houwen, F.; Verstraete, W. Agricultural factors affecting methane oxidation in arable soil. Biol. Fertil. Soils 1996, 21, 95–102. [Google Scholar] [CrossRef]
- Fabra, A.; Duffard, R.; De Duffard, A.E. Toxicity of 2, 4-dichlorophenoxyacetic acid to Rhizobium sp in pure culture. Bull. Environ. Contam. Toxicol. 1997, 59, 645–652. [Google Scholar] [CrossRef]
- Chalam, A.V.; Sasikala, C.; Ramana, C.V.; Uma, N.R.; Rao, P.R. Effect of pesticides on the diazotrophic growth and nitrogenase activity of purple nonsulfur bacteria. Bull. Environ. Contam. Toxicol. 1997, 58, 463–468. [Google Scholar] [CrossRef]
- Santos, A.; Flores, M. Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria. Lett. Appl. Microbiol. 1995, 20, 349–352. [Google Scholar] [CrossRef]
- Guo, P.; Zhu, L.; Wang, J.; Wang, J.; Xie, H.; Lv, D. Enzymatic activities and microbial biomass in black soil as affected by azoxystrobin. Environ. Earth Sci. 2015, 74, 1353–1361. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Gupta, S.; Varghese, E. Degradation of metaflumizone in soil: Impact of varying moisture, light, temperature, atmospheric CO2 level, soil type and soil sterilization. Chemosphere 2013, 90, 729–736. [Google Scholar] [CrossRef]
- Wightwick, A.M.; Reichman, S.M.; Menzies, N.W.; Allinson, G. The effects of copper hydroxide, captan and trifloxystrobin fungicides on soil phosphomonoesterase and urease activity. Water Air, Soil Pollut. 2013, 224, 1703. [Google Scholar] [CrossRef]
- Baćmaga, M.; Kucharski, J.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with azoxystrobin. Environ. Monit. Assess. 2015, 187, 615. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The effect of the Falcon 460 EC fungicide on soil microbial communities, enzyme activities and plant growth. Ecotoxicology 2016, 25, 1575–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmention: A review. Int. J. Environ. Bioremediation Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef]
- Saha, A.; Pipariya, A.; Bhaduri, D. Enzymatic activities and microbial biomass in peanut field soil as affected by the foliar application of tebuconazole. Environ. Earth Sci. 2016, 75, 558. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kohli, S.K.; Thukral, A.K.; Bhardwaj, R. Phytochemicals in Brassica juncea L. seedlings under imidacloprid-epibrassinolide treatment using GC-MS. J. Chem. Pharm. Res. 2015, 7, 708–711. [Google Scholar]
- Parween, T.; Jan, S.; Fatma, T. Alteration in nitrogen metabolism and plant growth during different developmental stages of green gram (Vigna radiata L.) in response to chlorpyrifos. Acta Physiol. Plant. 2011, 33, 2321–2328. [Google Scholar] [CrossRef]
- Parween, T.; Jan, S.; Fatma, T. Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int. J. Environ. Sci. Technol. 2012, 9, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, V.; Singh, R.; Thukral, A.K.; Bhardwaj, R. 24-Epibrassinolide induces the synthesis of phytochemicals effected by imidacloprid pesticide stress in Brassica juncea L. J. Pharmacogn. Phytochem. 2015, 4, 60–64. [Google Scholar]
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P.J. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Kaya, A.; Yigit, E. The physiological and biochemical effects of salicylic acid on sunflowers (Helianthus annuus) exposed to flurochloridone. Ecotoxicol. Environ. Saf. 2014, 106, 232–238. [Google Scholar] [CrossRef]
- Kaya, A.; Doganlar, Z.B. Exogenous jasmonic acid induces stress tolerance in tobacco (Nicotiana tabacum) exposed to imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef]
- Fernandes, B.; Soares, C.; Braga, C.; Rebotim, A.; Ferreira, R.; Ferreira, J.; Fidalgo, F.; Pereira, R.; Cachada, A. Ecotoxicological Assessment of a Glyphosate-Based Herbicide in Cover Plants: Medicago sativa L. as a Model Species. Appl. Sci. 2020, 10, 5098. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; Hu, M.; Liu, J. Biodegradation of fenvalerate and 3-phenoxybenzoic acid by a novel Stenotrophomonas sp. strain ZS-S-01 and its use in bioremediation of contaminated soils. Appl. Microbiol. Biotechnol. 2011, 90, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Jezierska-Tys, S.; Rutkowska, A. Soil response to chemicals used in a field experiment. Int. Agrophys. 2013, 27, 151–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, X.; Yu, G.; Wang, J.; Wu, J.; Wang, M.; Yang, Y.; Shi, K.; Yu, Y.; Chen, Z. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015, 5, 9018. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Huang, Y.Y.; Wang, L.; Huang, L.F.; Yu, Y.L.; Zhou, Y.H.; Yu, J.Q. Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pestic. Biochem. Physiol. 2006, 86, 42–48. [Google Scholar] [CrossRef]
- Ijaz, M.; Mahmood, K.; Honermeier, B. Interactive role of fungicides and plant growth regulator (Trinexapac) on seed yield and oil quality of winter rapeseed. Agronomy 2015, 5, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Ahmed, B.; Zaidi, A.; Khan, M.S. Toxicity of fungicides to Pisum sativum: A study of oxidative damage, growth suppression, cellular death and morpho-anatomical changes. RSC Adv. 2018, 8, 38483–38498. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M.; Khan, M.S. Assessment of plant growth promoting activities of rhizobacterium Pseudomonas putida under insecticide-stress. Microbiol. J. 2011, 2, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M.; Khan, M.S. Comparative toxicity of selected insecticides to pea plants and growth promotion in response to insecticide-tolerant and plant growth promoting Rhizobium leguminosarum. Crop Prot. 2010, 29, 325–329. [Google Scholar] [CrossRef]
- Saladin, G.; Clément, C. Physiological Side Effects of Pesticides on Non-target Plants. In Agriculture and Soil Pollution: New Research; Livingston, J.V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2005; pp. 53–86. [Google Scholar]
- Petit, A.N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Sáez, F.; Pozo, C.; Gómez, M.A.; Martínez-Toledo, M.V.; Rodelas, B.; Gónzalez-López, J. Growth and denitrifying activity of Xanthobacter autotrophicus CECT 7064 in the presence of selected pesticides. Appl. Microbiol. Biotechnol. 2006, 71, 563–567. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Qian, Y.; Zhao, X.; Wang, Q. The synergistic toxicity of the multiple chemical mixtures: Implications for risk assessment in the terrestrial environment. Environ. Int. 2015, 77, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Qian, Y.; Zhao, X.; Wang, Q. Ternary toxicological interactions of insecticides, herbicides, and a heavy metal on the earthworm Eisenia fetida. J. Hazard. Mater. 2015, 284, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Lin, Q.; He, Y.F.; Tian, G.M. Behavior of Cu and Zn under combined pollution of 2,4-dichlorophenol in the planted soil. Plant Soil 2004, 261, 127–134. [Google Scholar] [CrossRef]
- Chao, L.; Zhou, Q.X.; Chen, S.; Cui, S.; Wang, M.E. Single and joint stress of acetochlor and Pb on three agricultural crops in northeast China. J. Environ. Sci. 2007, 19, 719–724. [Google Scholar] [CrossRef]
- Divisekara, T.; Navaratne, A.N.; Abeysekara, A.S.K. Impact of a commercial glyphosate formulation on adsorption of Cd(II) and Pb(II) ions on paddy soil. Chemosphere 2018, 198, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, Q. Single and Binary-Combined Toxicity of Methamidophos, Acetochlor and Copper Acting on Earthworms Esisenia Foelide. Bull. Environ. Contam. Toxicol. 2003, 71, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, J.; Chu, Y.; Sun, C.; Chen, C.; Wang, Q. Combined effect of cypermethrin and copper on catalase activity in soil. J. Soils Sediments 2008, 8, 327–332. [Google Scholar] [CrossRef]
- García-Gómez, C.; Babín, M.; García, S.; Almendros, P.; Pérez, R.A.; Fernández, M.D. Joint effects of zinc oxide nanoparticles and chlorpyrifos on the reproduction and cellular stress responses of the earthworm Eisenia andrei. Sci. Total Environ. 2019, 688, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiong, Z.T. Toxic effects of cadmium, acetochlor and bensulfuron-methyl on nitrogen metabolism and plant growth in rice seedlings. Pestic. Biochem. Physiol. 2009, 94, 64–67. [Google Scholar] [CrossRef]
- Liu, N.; Zhong, G.; Zhou, J.; Liu, Y.; Pang, Y.; Cai, H.; Wu, Z. Separate and combined effects of glyphosate and copper on growth and antioxidative enzymes in Salvinia natans (L.) All. Sci. Total Environ. 2019, 655, 1448–1456. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Abouziena, H.F.; Elmergawi, R.A.; Sharma, S.; Omar, A.A.; Singh, M. Zinc Antagonizes Glyphosate Efficacy on Yellow Nutsedge ( Cyperus esculentus ). Weed Sci. 2009, 57, 16–20. [Google Scholar] [CrossRef]
- Khlifi, R.; Hamza-Chaffai, A. Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: A review. Toxicol. Appl. Pharmacol. 2010, 248, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, M.S.; Kumar, R. Contaminant of Heavy Metals in Groundwater & its Toxic Effects on Human Health & Environment. Int. J. Environ. Sci. Nat. Resour. 2019, 18, 555996. [Google Scholar] [CrossRef]
- Jiang, X.; Zou, B.; Feng, H.; Tang, J.; Tu, Y.; Zhao, X. Spatial distribution mapping of Hg contamination in subclass agricultural soils using GIS enhanced multiple linear regression. J. Geochemical Explor. 2019, 196, 1–7. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, D.; Ali, A.; Mi, S.; Liu, T.; Ren, C.; Li, R.; Zhang, Z. Accumulation, ecological-health risks assessment, and source apportionment of heavy metals in paddy soils: A case study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Navas-Acien, A.; Mark, D.B.; Lee, K.L. Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J. Am. Coll. Cardiol. 2016, 67, 2411–2418. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human health risk assessment of heavy metals in urban stormwater. Sci. Total Environ. 2016, 557, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guan, Q.; Tian, J.; Lin, J.; Yang, Y.; Yang, L.; Pan, N. Contamination characteristics, source apportionment, and health risk assessment of heavy metals in agricultural soil in the Hexi Corridor. CATENA 2020, 191, 104573. [Google Scholar] [CrossRef]
- Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef]
- Chunhabundit, R. Cadmium exposure and potential health risk from foods in contaminated area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef]
- Schoeters, G.; HOND, E.D.E.N.; Zuurbier, M.; Naginiene, R.; Van den Hazel, P.; Stilianakis, N.; Ronchetti, R.; Koppe, J.G. Cadmium and children: Exposure and health effects. Acta Paediatr. 2006, 95, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sherief, L.M.; Abdelkhalek, E.R.; Gharieb, A.F.; Sherbiny, H.S.; Usef, D.M.; Almalky, M.A.A.; Kamal, N.M.; Salama, M.A.; Gohar, W. Cadmium status among pediatric cancer patients in Egypt. Medicine (Baltimore) 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Kippler, M.; Tofail, F.; Bottai, M.; Hamadani, J.; Grandér, M.; Nermell, B.; Palm, B.; Rasmussen, K.M.; Vahter, M. Environmental exposure to metals and children’s growth to age 5 years: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. The Bioinorganic Chemistry of Lead in the Context of Its Toxicity. In Lead: Its Effects on Environment and Health; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; De Gruyter: Berlin, Germany, 2017; Volume 17, pp. 1–20. [Google Scholar]
- McMichael, J.R.; Stoff, B.K. Surma eye cosmetic in Afghanistan: A potential source of lead toxicity in children. Eur. J. Pediatr. 2018, 177, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.; Hryhorczuk, D.; Lanphear, B.P.; Rankin, K.M.; Lewis, D.A.; Forst, L.; Rosenberg, D. The impact of low-level lead toxicity on school performance among children in the Chicago Public Schools: A population-based retrospective cohort study. Environ. Health 2015, 14, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfadenhauer, L.M.; Burns, J.; Rohwer, A.; Rehfuess, E.A. A protocol for a systematic review of the effectiveness of interventions to reduce exposure to lead through consumer products and drinking water. Syst. Rev. 2014, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, W.T. Nutritional neuroscience. In Nutritional Neuroscience; Lieberman, H.R., Kanarek, R.B., Prasad, C., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2005; pp. 289–305. [Google Scholar]
- Zhou, G.; Ji, X.; Cui, N.; Cao, S.; Liu, C.; Liu, J. Association between serum copper status and working memory in schoolchildren. Nutrients 2015, 7, 7185–7196. [Google Scholar] [CrossRef]
- Roberts, E.A.; Socha, P. Wilson disease in children. In Handbook of clinical neurology; Członkowska, A., Schilsky, M.L., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 142, pp. 141–156. [Google Scholar]
- Arsenault, J.E.; Brown, K.H. Zinc intake of US preschool children exceeds new dietary reference intakes. Am. J. Clin. Nutr. 2003, 78, 1011–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J.L.; Piñero, D.J.; Parekh, N. Zinc and cognitive development in children: Perspectives from international studies. Top. Clin. Nutr. 2009, 24, 130–138. [Google Scholar] [CrossRef]
- Shaikhkhalil, A.K.; Curtiss, J.; Puthoff, T.D.; Valentine, C.J. Enteral zinc supplementation and growth in extremely-low-birth-weight infants with chronic lung disease. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 183. [Google Scholar] [CrossRef] [Green Version]
- Jeejeebhoy, K. Zinc: An essential trace element for parenteral nutrition. Gastroenterology 2009, 137, S7–S12. [Google Scholar] [CrossRef]
- Lim, K.H.C.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Excessive intake of zinc impairs immune responses. JAMA 1984, 252, 1443–1446. [Google Scholar] [CrossRef]
- Willoughby, J.L.; Bowen, C.N. Zinc deficiency and toxicity in pediatric practice. Curr. Opin. Pediatr. 2014, 26, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Avenant-Oldewage, A.; Marx, H.M. Bioaccumulation of chromium, copper and iron in the organs and tissues of Clarias gariepinus in the Olifants River, Kruger National Park. Water SA 2000, 26, 569–582. [Google Scholar]
- Jiang, L.-F.; Yao, T.-M.; Zhu, Z.-L.; Wang, C.; Ji, L.-N. Impacts of Cd (II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim. Biophys. Acta-Proteins Proteomics 2007, 1774, 1414–1421. [Google Scholar] [CrossRef]
- Reyes-Hinojosa, D.; Lozada-Pérez, C.A.; Cuevas, Y.Z.; López-Reyes, A.; Martínez-Nava, G.; Fernández-Torres, J.; Olivos-Meza, A.; Landa-Solis, C.; Gutiérrez-Ruiz, M.C.; Del Castillo, E.R. Toxicity of cadmium in musculoskeletal diseases. Environ. Toxicol. Pharmacol. 2019, 72, 103219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Vasallo, M.D.; Aragonés, N.; Pollan, M.; López-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef] [Green Version]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.N.; Zeb, N. Assessment of environmental contamination using feathers of Bubulcus ibis L., as a biomonitor of heavy metal pollution, Pakistan. Ecotoxicology 2009, 18, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A.; Clarkson, T.W. Toxic effects of metals. In Casarett and Doull’s Toxicology: The Basic Science of Poisons; Klaassen, C.D., Ed.; McGraw-Hill Medical: New York, NY, USA, 2001; pp. 811–867. [Google Scholar]
- Ogwuegbu, M.O.; Ijioma, M.A. Effects of certain heavy metals on the population due to mineral exploitation. In Proceedings of the International Conference on Scientific and Environmental Issues in the Population, Environment and Sustainable Development in Nigeria, University of Ado Ekiti, Ado Ekiti, Ekiti State, Nigeria, 10 October 2003; pp. 8–10. [Google Scholar]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Gamakaranage, C.S.S.K.; Rodrigo, C.; Weerasinghe, S.; Gnanathasan, A.; Puvanaraj, V.; Fernando, H. Complications and management of acute copper sulphate poisoning; a case discussion. J. Occup. Med. Toxicol. 2011, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. BioMetals 2014, 27, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.D. Cellular copper transport and metabolism. Annu. Rev. Nutr. 2000, 20, 291–310. [Google Scholar] [CrossRef]
- Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell Res. 2016, 347, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M. Zinc. In Trace Elements in Human and Animal Nutrition; Mertz, W., Ed.; Academic Press, Elsevier: Orlando, FL, USA, 1986; pp. 13–19. [Google Scholar]
- Morris, D.R.; Levenson, C.W. Neurotoxicity of Zinc. In Neurotoxicity of Metals; Aschner, M.C.L., Ed.; Springer: Cham, Switzerland, 2017; pp. 303–312. [Google Scholar]
- Bush, A.I. The metal theory of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33, S277–S281. [Google Scholar] [CrossRef] [PubMed]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy metal exposure and metabolic syndrome: Evidence from human and model system studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, T.; Tao, Y.; Feng, Y.; Shen, X.; Mei, S. Exposure to organochlorine pesticides and non-Hodgkin lymphoma: A meta-analysis of observational studies. Sci. Rep. 2016, 6, 25768. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.E.; Meade, B.J. Potential Health Effects Associated with Dermal Exposure to Occupational Chemicals. Environ. Health Insights 2014, 8s1, EHI.S15258. [Google Scholar] [CrossRef] [Green Version]
- Fareed, M.; Kesavachandran, C.N.; Pathak, M.K.; Bihari, V.; Kuddus, M.; Srivastava, A.K. Visual disturbances with cholinesterase depletion due to exposure of agricultural pesticides among farm workers. Toxicol. Environ. Chem. 2012, 94, 1601–1609. [Google Scholar] [CrossRef]
- Amaral, A.F.S. Pesticides and Asthma: Challenges for Epidemiology. Front. Public Health 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Freeman, L.E.B.; Hoppin, J.A.; Koutros, S.; Sandler, D.P.; Lynch, C.F.; Hines, C.J.; Thomas, K.; Blair, A.; Alavanja, M.C.R. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polanco Rodríguez, Á.G.; Riba López, M.I.; DelValls Casillas, T.Á.; Araujo León, J.A.; Mahjoub, O.; Prusty, A.K. Monitoring of organochlorine pesticides in blood of women with uterine cervix cancer. Environ. Pollut. 2017, 220, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Parrón, T.; Alarcón, R. Pesticides and asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 90–96. [Google Scholar] [CrossRef]
- Azandjeme, C.; Bouchard, M.; Fayomi, B.; Djrolo, F.; Houinato, D.; Delisle, H. Growing Burden of Diabetes in Sub- Saharan Africa: Contribution of Pesticides? Curr. Diabetes Rev. 2013, 9, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Koifman, S. NeuroToxicology Pesticide exposure and Parkinson ’ s disease: Epidemiological evidence of association. Neurotoxicology 2012, 33, 947–971. [Google Scholar] [CrossRef]
- Brouwer, M.; Huss, A.; van der Mark, M.; Nijssen, P.C.G.; Mulleners, W.M.; Sas, A.M.G.; van Laar, T.; de Snoo, G.R.; Kromhout, H.; Vermeulen, R.C.H. Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ. Int. 2017, 107, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Frazier, L.M. Reproductive Disorders Associated with Pesticide Exposure. J. Agromedicine 2007, 12, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mehrpour, O.; Karrari, P.; Zamani, N.; Tsatsakis, A.M.; Abdollahi, M. Occupational exposure to pesticides and consequences on male semen and fertility: A review. Toxicol. Lett. 2014, 230, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Garry, V.F. Pesticides and children. Toxicol. Appl. Pharmacol. 2004, 198, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Danadevi, K.; Mahboob, M.; Rozati, R.; Banu, B.S.; Rahman, M.F. Evaluation of genetic damage in workers employed in pesticide production utilizing the Comet assay. Mutagenesis 2003, 18, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Peluso, M.; Merlo, F.; Munnia, A.; Bolognesi, C.; Puntoni, R.; Parodi, S. 32P-postlabeling detection of DNA adducts in peripheral white blood cells of greenhouse floriculturists from Western Liguria, Italy. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 361–369. [Google Scholar] [PubMed]
- Edwards, T.M.; Myers, J.P. Environmental Exposures and Gene Regulation in Disease Etiology. Environ. Health Perspect. 2007, 115, 1264–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonell, E.; Xamena, N.; Creus, A.; Marcos, R. Cytogenetic biomonitoring in a Spanish group of agricultural workers exposed to pesticides. Mutagenesis 1993, 8, 511–517. [Google Scholar] [CrossRef]
- Dong, L.M.; Potter, J.D.; White, E.; Ulrich, C.M.; Cardon, L.R.; Peters, U. Genetic Susceptibility to Cancer: The role of polymorphisms in candidate genes. JAMA 2008, 299, 2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.R.; Buha Djordjevic, A. Heavy metal and pesticide exposure: A mixture of potential toxicity and carcinogenicity. Curr. Opin. Toxicol. 2020, 19, 72–79. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Cabaj, M.; Massanyi, P.; Martiniakova, M.; Omelka, R.; Krajcovicova, V.; Duranova, H. Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Guo, W.; Qian, Y.; Zhang, S.; Ren, D.; Liu, S. Synergistic hepatotoxicity by cadmium and chlorpyrifos: Disordered hepatic lipid homeostasis. Mol. Med. Rep. 2015, 12, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.; Choi, S.; Kim, K.; Kim, S.M.; Park, S.M. Association between urban green space and the risk of cardiovascular disease: A longitudinal study in seven Korean metropolitan areas. Environ. Int. 2019, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]







| Metals | Basaltic Igneous | Granite Igneous | Shales and Clays | Black Shales | Sandstone |
|---|---|---|---|---|---|
| Cd | 0.006–0.6 | 0.003–0.18 | 0.0–11 | <0.3–8.4 | - |
| Pb | 30–160 | 4–30 | 18–120 | 20–200 | - |
| Cu | 48–240 | 5–140 | 18–180 | 34–1500 | 2–41 |
| Zn | 2–18 | 6–30 | 16–50 | 7–150 | <1–31 |
| Heavy Metals | P Fertilizers | N Fertilizers | Lime Fertilizers | Manure Fertilizers | ||||
|---|---|---|---|---|---|---|---|---|
| Worldwide | EU | Worldwide | EU | Worldwide | EU | Worldwide | EU | |
| Cd | 0.1–170 | 13 | 0.05–8.5 | 0.9 | 0.04–.01 | 0.2 | 0.3–0.8 | – |
| Pb | 1.0–300 | 26 | 1.0–15 | 2.0 | 2.0–125 | 5.6 | 2.0–60 | – |
| Cu | 7.0–225 | 13 | 2–1450 | 1.9 | 20–1250 | 8.2 | 6.6–350 | – |
| Zn | 50–1450 | 236 | 1.0–42 | 5.0 | 10–450 | 22 | 15–250 | – |
| Mode of Entry | Definition | Example |
|---|---|---|
| Contact pesticides | They enter the target’s body by direct contact (especially the physical contact). This type of pesticide enters the body via the epidermal layer. | Diquat dibromide |
| Systemic pesticides | They are absorbed by the plant vascular system, then translocate to the remaining untreated tissues. | Glyphosate |
| Stomach poisons | They enter the target’s body via their digestive tract during their food ingestion, followed by death due to the poisoning. | Malathion |
| Repellents | They do not enter the target’s body and kill them; they only push back and resist the pests to keep them away from the host. | Methyl anthranilate |
| Fumigants | They kill the pests by producing vapor (gaseous state) of the pesticide. These vapors enter the pest’s body through the spiracles (tracheal system). | 1,3-dichloropropene |
| WHO Type | Toxicity Level | LD50 for the Rat(mg/kg Body Weight) | Examples | |
|---|---|---|---|---|
| Oral | Dermal | |||
| Type Ia | Extremely hazardous | <5 | <50 | Parathion, Dieldrin |
| Type Ib | Highly hazardous | 5–50 | 50–200 | Eldrin, Dichlorvos |
| Type II | Moderately hazardous | 50–2000 | 200–2000 | DDT, Chlordane |
| Type III | Slightly hazardous | >2000 | >2000 | Malathion |
| Heavy Metals | Toxicity Form | Toxic Effects | References | |
|---|---|---|---|---|
| Soil | Plant | |||
| Cd | Cd+2 | Kill microorganisms, absorb organic matter, and change soil physicochemical characteristics. | Reduce biomass and root length, inhibit seed germination, and reduce stem conductivity. | [70,99,107,134,135] |
| Pb | Pb+2 | Change soil pH, affect soil sorption capacity, and reduce soil fertility. | DNA damage, decrease chlorophyll content, decrease protein content, and cause stunted foliage. | [32,74,135,136] |
| Cu | Cu salts | Change urease activity, affect microbial communities, and decrease oxidation potential. | Root deformation, decrease shoot length, reduce polypeptides, and change in lipid content. | [82,86,135,137] |
| Zn | Zn+2 | Change bicarbonate and organic matter content, inhibit enzymatic activity, and affect soil pH. | Variation in enzymatic activity, obstruction of elements transmission, and cause interveinal chlorosis. | [93,135,138,139] |
| Pesticide Type | Toxic Effects | References | |
|---|---|---|---|
| Soil | Plant | ||
| Insecticides | Destruction of microbial structural proteins, symbiotic attributes reduction, change soil chemistry and enzymatic activity. | Reduction in grain protein content, blockage of stomatal conductance, and alterations in the photosynthetic process. | [192,193] |
| Herbicides | Reduction of the soil nutrients availability and suppression of phosphatase and nitrogenase activities. | Alteration of the physiological and biochemical plant efficiency, increasing the susceptibility of plants toward diseases. | [156,194] |
| Fungicides | Interruption of phosphatase, urease, and dehydrogenase activities and inhibition of the nitrifying bacterial growth. | Reduction of chlorophyll and carotenoid concentrations, destruction of chloroplasts, stomatal closure, and electron transfer suppression. | [195,196,197] |
| Heavy Metal | Pesticide | Joint Interaction | Joint Toxic Effect | References |
|---|---|---|---|---|
| Soil organisms | ||||
| Cd | λ-cyhalothrin Chlorpyrifos, Atrazine | Antagonism Synergism | Earthworm mortality. | [200] |
| Pb | Acetochlor Glyphosate | Synergism Antagonism | Na Change soil pH. | [202,203] |
| Cu | Acetochlor Cypermethrin | Synergism Synergism & Antagonism | Earthworm mortality. Change catalase activity. | [204,205] |
| Zn | Chlorpyrifos 2,4-DCP | Synergism Antagonism | Reduce acetylcholinesterase activity. Limited effect on Zn dissolution. | [201,206] |
| Plant parts | ||||
| Cd | Acetochlor Bensulfuron-methyl | Synergism Synergism | Decline soluble protein content, Affect nitrate reductase activity, suppression of roots and shoots growth. | [207] |
| Pb | Acetochlor | Synergism & Antagonism * | Root elongation inhibition. | [202] |
| Cu | Glyphosate | Synergism & Antagonism * | Change tissue structure of the cell membrane, severe production of ROS, membrane lipids peroxidation. | [208,209] |
| Zn | Glyphosate | Antagonism | Reduce the phytotoxicity of destructive weeds. | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. https://doi.org/10.3390/toxics9030042
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang M-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics. 2021; 9(3):42. https://doi.org/10.3390/toxics9030042
Chicago/Turabian StyleAlengebawy, Ahmed, Sara Taha Abdelkhalek, Sundas Rana Qureshi, and Man-Qun Wang. 2021. "Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications" Toxics 9, no. 3: 42. https://doi.org/10.3390/toxics9030042
APA StyleAlengebawy, A., Abdelkhalek, S. T., Qureshi, S. R., & Wang, M.-Q. (2021). Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics, 9(3), 42. https://doi.org/10.3390/toxics9030042