Microbial Population Dynamics in Model Sewage Treatment Plants and the Fate and Effect of Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gold Nanoparticles
2.2. Activated Sludge
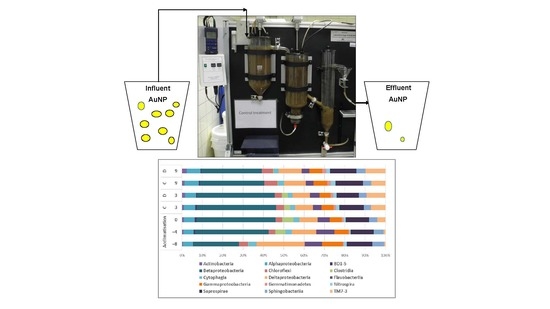
2.3. Model Sewage Treatment Plant
2.4. Microbial Community Analysis
2.5. Gold Analysis
3. Results
3.1. General Changes of Microbial Diversity
3.2. Au Analysis in Activated Sludge
3.3. Effect of AuNPs on Microbial Population
3.4. Effect of Dispersant and AuNPs on the STP Function
4. Discussion
4.1. General Change of Microbial Diversity over Time
4.2. Fate of AuNPs in the STP
4.3. Effect of AuNPs on Structural Diversity and Function of a STP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment. Chapter r.7b: Endpoint Specific Guidance Draft Version 4.0. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r7b_en.pdf/1a551efc-bd6a-4d1f-b719-16e0d3a01919 (accessed on 11 November 2020).
- OECD Guideline 303A. Oecd guidelines for the testing of chemicals. In Test Guideline 303: Simulation Test—Aerobic Sewage Treatment—A: Activated Sludge Units; B: Biofilms; Organisation for Economic Co-operation and Development: Paris, France, 2001. [Google Scholar]
- Meerbergen, K.; Van Geel, M.; Waud, M.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Assessing the composition of microbial communities in textile wastewater treatment plants in comparison with municipal wastewater treatment plants. MicrobiologyOpen 2017, 6, e00413. [Google Scholar] [CrossRef] [Green Version]
- Wolff, D.; Krah, D.; Dötsch, A.; Ghattas, A.-K.; Wick, A.; Ternes, T.A. Insights into the variability of microbial community composition and micropollutant degradation in diverse biological wastewater treatment systems. Water Res. 2018, 143, 313–324. [Google Scholar] [CrossRef]
- Lee, S.; Geller, J.T.; Torok, T.; Wu, C.H.; Singer, M.; Reid, F.C.; Tarjan, D.R.; Hazen, T.C.; Arkin, A.P.; Hillson, N.J. Characterization of wastewater treatment plant microbial communities and the effects of carbon sources on diversity in laboratory models. PLoS ONE 2014, 9, e105689. [Google Scholar] [CrossRef]
- Moretti, G.; Matteucci, F.; Ercole, C.; Vegliò, F.; Del Gallo, M. Microbial community distribution and genetic analysis in a sludge active treatment for a complex industrial wastewater: A study using microbiological and molecular analysis and principal component analysis. Ann. Microbiol. 2016, 66, 397–405. [Google Scholar] [CrossRef]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment Appendix r7-1 for Nanomaterials Applicable to Chapter r7b Endpoint Specific Guidance Version 2.0. Available online: https://echa.europa.eu/documents/10162/13632/appendix_r7b_nanomaterials_en.pdf/6eca425a-ede1-4c9e-8151-af77973caf32 (accessed on 17 October 2020).
- Ren, S.; Frymier, P.D. Use of multidimensional scaling in the selection of wastewater toxicity test battery components. Water Res. 2003, 37, 1655–1661. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward nanotechnology-enabled approaches against the covid-19 pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- García-Cambero, J.P.; Núñez García, M.; López, G.D.; Herranz, A.L.; Cuevas, L.; Pérez-Pastrana, E.; Cuadal, J.S.; Castelltort, M.R.; Calvo, A.C. Converging hazard assessment of gold nanoparticles to aquatic organisms. Chemosphere 2013, 93, 1194–1200. [Google Scholar] [CrossRef]
- Harper, S.; Usenko, C.; Hutchison, J.E.; Maddux, B.L.S.; Tanguay, R.L. In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalisation and route of exposure. J. Exp. Nanosci. 2008, 3, 195–206. [Google Scholar] [CrossRef]
- Browning, L.M.; Lee, K.J.; Huang, T.; Nallathamby, P.D.; Lowman, J.E.; Nancy Xu, X.-H. Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale 2009, 1, 138. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Chen, Z.; Qiu, Z.; Li, Y.; Laing, G.D.; Liu, A.; Yan, C. Gold and silver nanoparticle effects on ammonia-oxidizing bacteria cultures under ammoxidation. Chemosphere 2015, 120, 737–742. [Google Scholar] [CrossRef]
- García, A.; Delgado, L.; Torà, J.A.; Casals, E.; González, E.; Puntes, V.; Font, X.; Carrera, J.; Sánchez, A. Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment. J. Hazard. Mater. 2012, 199–200, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BMU. Federal ministry for the environment, nature conservation and nuclear safety. Verordnung zur neuordnung der klärschlammverwertung. Bundesgesetzblatt 2017, 65, 3465. [Google Scholar]
- Mahapatra, I.; Sun, T.Y.; Clark, J.R.; Dobson, P.J.; Hungerbuehler, K.; Owen, R.; Nowack, B.; Lead, J. Probabilistic modelling of prospective environmental concentrations of gold nanoparticles from medical applications as a basis for risk assessment. J. Nanobiotechnol. 2015, 13, 93. [Google Scholar] [CrossRef] [Green Version]
- DIN EN 16174:2012-11. Sludge, Treated Biowaste and Soil—Digestion of Aqua Regia Soluble Fractions of Elements; German Version En 16174:2012; Beuth: Berlin, Germany, 2012. [Google Scholar]
- Wagner, M.; Amann, R.; Lemmer, H.; Schleifer, K.H. Probing activated sludge with oligonucleotides specific for proteobacteria: Inadequacy of culture-dependent methods for describing microbial community structure. Appl. Environ. Microbiol. 1993, 59, 1520–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snaidr, J.; Amann, R.; Huber, I.; Ludwig, W.; Schleifer, K.H. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl. Environ. Microbiol. 1997, 63, 2884–2896. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, A.L.; Souza, A.J.; Andrade, P.A.M.; Andreote, F.D.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Sewage sludge microbial structures and relations to their sources, treatments, and chemical attributes. Front. Microbiol. 2018, 9, 1462. [Google Scholar] [CrossRef]
- Xu, S.; Yao, J.; Ainiwaer, M.; Hong, Y.; Zhang, Y. Analysis of bacterial community structure of activated sludge from wastewater treatment plants in winter. BioMed Res. Int. 2018, 2018, 8278970. [Google Scholar] [CrossRef] [Green Version]
- Juretschko, S.; Loy, A.; Lehner, A.; Wagner, M. The microbial community composition of a nitrifying-denitrifying activated sludge from an industrial sewage treatment plant analyzed by the full-cycle rrna approach. Syst. Appl. Microbiol. 2002, 25, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Wagner, M. Nitrospira. Trends Microbiol. 2018, 26, 462–463. [Google Scholar] [CrossRef]
- Geets, J.; de Cooman, M.; Wittebolle, L.; Heylen, K.; Vanparys, B.; De Vos, P.; Verstraete, W.; Boon, N. Real-time pcr assay for the simultaneous quantification of nitrifying and denitrifying bacteria in activated sludge. Appl. Microbiol. Biotechnol. 2007, 75, 211–221. [Google Scholar] [CrossRef]
- Rezaee, A.; Naimi, N.; Hashemi, S.E.; Hosseini, H.; Darvishi Cheshmeh Soltani, R. Molecular identification of nitrifying bacteria in activated sludge. J. Mater. Environ. Sci. 2013, 4, 601–604. [Google Scholar]
- Johnston, J.; LaPara, T.; Behrens, S. Composition and dynamics of the activated sludge microbiome during seasonal nitrification failure. Sci. Rep. 2019, 9, 4565. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.K.; Kleerebezem, R.; de Bruin, L.M.; Verheijen, P.J.; Abbas, B.; Habermacher, J.; van Loosdrecht, M.C. Microbial diversity differences within aerobic granular sludge and activated sludge flocs. Appl. Microbiol. Biotechnol. 2013, 97, 7447–7458. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Q.; Yan, G.; Zhu, H.; Xu, X.Y.; Zhu, L. Seasonal bacterial community succession in four typical wastewater treatment plants: Correlations between core microbes and process performance. Sci. Rep. 2018, 8, 4566. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Xiang, F.; Zhao, L.; Qiao, Z. Activated sludge microbial community and treatment performance of wastewater treatment plants in industrial and municipal zones. Int. J. Environ. Res. Public Health 2020, 17, 436. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Zhang, Y.; Cui, C.; Zheng, S. Effect of cod level and hrt on microbial community in a yeast-predominant activated sludge system. Bioresour. Technol. 2010, 101, 3463–3465. [Google Scholar] [CrossRef]
- Ayala-Del-Río, H.L.; Callister, S.J.; Criddle, C.S.; Tiedje, J.M. Correspondence between community structure and function during succession in phenol- and phenol-plus-trichloroethene-fed sequencing batch reactors. Appl. Environ. Microbiol. 2004, 70, 4950–4960. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Ji, F.Y.; Fu, X.F.; Chen, Q.K. Effects of gas/water ratio on the characteristics of nitrogen removal and the microbial community in post solid-phase denitrification biofilter process. Huan Jing ke Xue = Huanjing Kexue 2018, 39, 3297–3305. [Google Scholar]
- Miura, Y.; Watanabe, Y.; Okabe, S. Membrane biofouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater: Impact of biofilm formation. Environ. Sci. Technol. 2007, 41, 632–638. [Google Scholar] [CrossRef]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial community evenness favours functionality under selective stress. Nature 2009, 458, 623–626. [Google Scholar] [CrossRef]
- Li, W.M.; Liao, X.W.; Guo, J.S.; Zhang, Y.X.; Chen, Y.P.; Fang, F.; Yan, P. New insights into filamentous sludge bulking: The potential role of extracellular polymeric substances in sludge bulking in the activated sludge process. Chemosphere 2020, 248, 11. [Google Scholar] [CrossRef]
- Schlich, K.; Klawonn, T.; Terytze, K.; Hund-Rinke, K. Hazard assessment of a silver nanoparticle in soil applied via sewage sludge. Environ. Sci. Eur. 2013, 25, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Chaúque, E.; Zvimba, J.; Ngila, J.; Musee, N. Fate, behaviour, and implications of zno nanoparticles in a simulated wastewater treatment plant. Water SA 2016, 42, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Kiser, M.A.; Westerhoff, P.; Benn, T.; Wang, Y.; Pérez-Rivera, J.; Hristovski, K. Titanium nanomaterial removal and release from wastewater treatment plants. Environ. Sci. Technol. 2009, 43, 6757–6763. [Google Scholar] [CrossRef] [PubMed]
- Limbach, L.K.; Bereiter, R.; Müller, E.; Krebs, R.; Gälli, R.; Stark, W.J. Removal of oxide nanoparticles in a model wastewater treatment plant: Influence of agglomeration and surfactants on clearing efficiency. Environ. Sci. Technol. 2008, 42, 5828–5833. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Voegelin, A.; Ort, C.; Sinnet, B.; Thalmann, B.; Krismer, J.; Hagendorfer, H.; Elumelu, M.; Mueller, E. Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res. 2013, 47, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Metch, J.W.; Burrows, N.D.; Murphy, C.J.; Pruden, A.; Vikesland, P.J. Metagenomic analysis of microbial communities yields insight into impacts of nanoparticle design. Nat. Nanotechnol. 2018, 13, 253–259. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bandyopadhyay, A.; Sarkar, K. Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. J. Nanobiotechnol. 2011, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial effect of gold nanoparticles against corynebacterium pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajerski, W.; Ochonska, D.; Brzychczy-Wloch, M.; Indyka, P.; Jarosz, M.; Golda-Cepa, M.; Sojka, Z.; Kotarba, A. Attachment efficiency of gold nanoparticles by gram-positive and gram-negative bacterial strains governed by surface charges. J. Nanopart. Res. 2019, 21, 186. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lutken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the rhodiola rosea rhizome extracts. Artif. Cell. Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by nio nanoparticles synthesized from eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Thomsen, T.R.; Nielsen, J.L. Bacterial composition of activated sludge—importance for floc and sludge properties. Water Sci. Technol. 2004, 49, 51–58. [Google Scholar] [CrossRef]
- Arvidsson, R.; Hansen, S.F.; Baun, A. Influence of natural organic matter on the aquatic ecotoxicity of engineered nanoparticles: Recommendations for environmental risk assessment. NanoImpact 2020, 20, 100263. [Google Scholar] [CrossRef]





| Sample Name | Au Concentration [mg/mL] | Hydrodynamic Diameter [nm] | Zeta Potential [mV] | Appearance | pH |
|---|---|---|---|---|---|
| Dispersant | - | - | Not applicable | Transparent fluid | 5.0 |
| AuNPs | 5 ± 0.5 | 25.2 ±0.6 | −15.2 ± 1.0 | Magenta fluid | 5.0 |
| Au in Sewage Sludge | Replicate 1 | Replicate 2 | Mean |
|---|---|---|---|
| Nominal Au [mg/g dm sludge] | 7.6 | 7.6 | 7.6 |
| Measured Au [mg/g dm sludge] | 6.8 | 3.0 | 4.9 |
| Percent of added Au in sludge | 90.0 | 39.2 | 64.6 |
| Measured Au [mg/L effluent] | 1.8 | 1.6 | 1.7 |
| Percent of added Au in effluent | 0.7 | 0.6 | 0.6 |
| Day | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Dispersant | Dispersant | Au-NP | |||||
| NH4 | SD | NH4 | SD | NH4 | SD | NH4 | SD | |
| [mg/L] | [mg/L] | [mg/L] | [mg/L] | |||||
| −6 | 13.0 | 4.2 | 11.5 | 0.7 | 25.0 | 9.9 | 19.5 | 6.4 |
| −4 | 6.7 | 8.9 | 0.4 | 0.0 | 5.6 | 6.2 | 3.4 | 3.7 |
| 0 | 0.3 | 0.3 | 0.2 | 0.1 | 0.5 | 0.2 | 0.5 | 0.4 |
| 3 | 0.5 | 0.4 | 0.3 | 0.1 | 0.6 | 0.4 | 0.5 | 0.1 |
| 6 | 0.2 | 0.1 | 0.1 | 0.0 | 0.2 | 0.1 | 0.7 | 0.4 |
| 9 | 0.1 | 0.0 | 0.4 | 0.4 | 0.3 | 0.0 | 0.5 | 0.2 |
| Day | NO2 | SD | NO2 | SD | NO2 | SD | NO2 | SD |
| [mg/L] | [mg/L] | [mg/L] | [mg/L] | |||||
| −6 | 6.0 | 0.3 | 9.5 | 1.3 | 8.0 | 1.8 | 5.0 | 2.1 |
| −4 | 6.7 | 4.8 | 11.3 | 0.5 | 20.8 | 4.0 | 15.5 | 1.3 |
| 0 | 6.4 | 6.7 | 8.6 | 1.2 | 16.0 | 0.6 | 11.0 | 2.8 |
| 3 | 5.3 | 5.6 | 3.2 | 1.5 | 17.4 | 0.3 | 12.1 | 2.4 |
| 6 | 1.3 | 0.4 | 1.0 | 0.0 | 12.3 | 2.7 | 5.5 * | 0.4 |
| 9 | 1.2 | 0.3 | 1.0 | 0.0 | 16.6 | 3.7 | 3.1 * | 0.7 |
| Day | NO3 | SD | NO3 | SD | NO3 | SD | NO3 | SD |
| [mg/L] | [mg/L] | [mg/L] | [mg/L] | |||||
| −6 | 15.5 | 9.2 | 19.5 | 2.1 | 27.5 | 13.4 | 32.0 | 8.5 |
| −4 | 18.5 | 14.8 | 22.0 | 5.7 | 24.5 | 10.6 | 32.0 | 11.3 |
| 0 | 26.5 | 9.2 | 31.0 | 1.4 | 31.5 | 12.0 | 43.0 | 22.6 |
| 3 | 27.5 | 9.2 | 44.5 | 0.7 | 29.0 | 5.7 | 46.5 | 19.1 |
| 6 | 20.5 | 2.1 | 24.5 | 0.7 | 30.0 | 5.7 | 50.5 | 14.8 |
| 9 | 25.0 | 2.8 | 21.0 | 2.8 | 32.0 | 4.2 | 54 * | 9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlich, K.; Díaz, C.; Gomez Pizarro, B.; Knopf, B.; Schlinkert, R.; Wege, F.F.; Jurack, A.; Hund-Rinke, K. Microbial Population Dynamics in Model Sewage Treatment Plants and the Fate and Effect of Gold Nanoparticles. Toxics 2021, 9, 54. https://doi.org/10.3390/toxics9030054
Schlich K, Díaz C, Gomez Pizarro B, Knopf B, Schlinkert R, Wege FF, Jurack A, Hund-Rinke K. Microbial Population Dynamics in Model Sewage Treatment Plants and the Fate and Effect of Gold Nanoparticles. Toxics. 2021; 9(3):54. https://doi.org/10.3390/toxics9030054
Chicago/Turabian StyleSchlich, Karsten, Cecilia Díaz, Benjamin Gomez Pizarro, Burkhard Knopf, Ruben Schlinkert, Franziska Frederike Wege, Anne Jurack, and Kerstin Hund-Rinke. 2021. "Microbial Population Dynamics in Model Sewage Treatment Plants and the Fate and Effect of Gold Nanoparticles" Toxics 9, no. 3: 54. https://doi.org/10.3390/toxics9030054
APA StyleSchlich, K., Díaz, C., Gomez Pizarro, B., Knopf, B., Schlinkert, R., Wege, F. F., Jurack, A., & Hund-Rinke, K. (2021). Microbial Population Dynamics in Model Sewage Treatment Plants and the Fate and Effect of Gold Nanoparticles. Toxics, 9(3), 54. https://doi.org/10.3390/toxics9030054