Vulnerability and Burden of All-Cause Mortality Associated with Particulate Air Pollution during COVID-19 Pandemic: A Nationwide Observed Study in Italy
Abstract
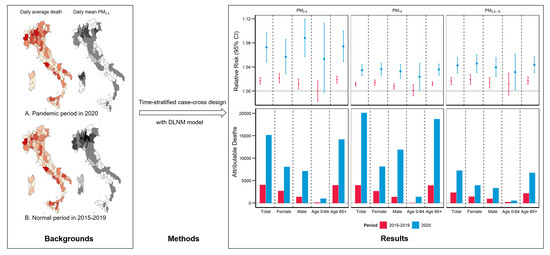
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mortality Data
2.3. Environmental Exposure Data
2.4. Statistical Analysis
2.5. Sensitivity Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.Y.; Guo, Y.; Markevych, I.; Qian, Z.M.; Bloom, M.S.; Heinrich, J.; Dharmage, S.C.; Rolling, C.A.; Jordan, S.S.; Komppula, M.; et al. Association of Long-term Exposure to Ambient Air Pollutants with Risk Factors for Cardiovascular Disease in China. JAMA Netw. Open 2019, 2, e190318. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Kong, S.; Chen, N.; Yan, Y.; Liu, D.; Zhu, B.; Xu, K.; Cao, W.; Ding, Q.; Lan, B.; et al. Significant changes in the chemical compositions and sources of PM2.5 in Wuhan since the city lockdown as COVID-19. Sci. Total. Environ. 2020, 739, 140000. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, M.; Huang, C.; Kinney, P.L.; Anastas, P.T. Air pollution reduction and mortality benefit during the COVID-19 outbreak in China. Lancet Planet. Health 2020, 4, e210–e212. [Google Scholar] [CrossRef]
- Otmani, A.; Benchrif, A.; Tahri, M.; Bounakhla, M.; Chakir, E.M.; El Bouch, M.; Krombi, M. Impact of Covid-19 lockdown on PM10, SO2 and NO2 concentrations in Salé City (Morocco). Sci. Total. Environ. 2020, 735, 139541. [Google Scholar] [CrossRef]
- Kanniah, K.D.; Zaman, N.A.F.K.; Kaskaoutis, D.G.; Latif, M.T. COVID-19’s impact on the atmospheric environment in the Southeast Asia region. Sci. Total. Environ. 2020, 736, 139658. [Google Scholar] [CrossRef]
- Gautam, S. The Influence of COVID-19 on Air Quality in India: A Boon or Inutile. Bull. Environ. Contam. Toxicol. 2020, 104, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Nakada, L.Y.K.; Urban, R.C. COVID-19 pandemic: Impacts on the air quality during the partial lockdown in São Paulo state, Brazil. Sci. Total. Environ. 2020, 730, 139087. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-Y.; Fong, K.C.; Heo, S.; Kim, H.; Lim, C.C.; Bell, M.L. Reductions in mortality resulting from reduced air pollution levels due to COVID-19 mitigation measures. Sci. Total. Environ. 2020, 744, 141012. [Google Scholar] [CrossRef]
- Tobías, A.; Carnerero, C.; Reche, C.; Massagué, J.; Via, M.; Minguillón, M.C.; Alastuey, A.; Querol, X. Changes in air quality during the lockdown in Barcelona (Spain) one month into the SARS-CoV-2 epidemic. Sci. Total Environ. 2020, 726, 138540. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, C.; Jackson, R.B.; Jones, M.W.; Smith, A.J.P.; Abernethy, S.; Andrew, R.M.; De-Gol, A.J.; Willis, D.R.; Shan, Y.; Canadell, J.G.; et al. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat. Clim. Chang. 2020, 10, 647–653. [Google Scholar] [CrossRef]
- Boccia, S.; Ricciardi, W.; Ioannidis, J.P.A. What Other Countries Can Learn From Italy During the COVID-19 Pandemic. JAMA Intern. Med. 2020, 180, 927. [Google Scholar] [CrossRef] [Green Version]
- Alicandro, G.; Remuzzi, G.; La Vecchia, C. Italy’s first wave of the COVID-19 pandemic has ended: No excess mortality in May, 2020. Lancet 2020, 396, e27–e28. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bertanza, G.; Pedrazzani, R.; Ricciardi, P.; Miino, M.C. Lockdown for CoViD-2019 in Milan: What are the effects on air quality? Sci. Total. Environ. 2020, 732, 139280. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Singh, R.P. Decline in PM2.5 concentrations over major cities around the world associated with COVID-19. Environ. Res. 2020, 187, 109634. [Google Scholar] [CrossRef]
- Martelletti, L.; Martelletti, P. Air Pollution and the Novel Covid-19 Disease: A Putative Disease Risk Factor. SN Compr. Clin. Med. 2020, 2, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, A.; Yin, J. Short-Term Effects of Ambient Ozone, PM(2.5,) and Meteorological Factors on COVID-19 Con-firmed Cases and Deaths in Queens, New York. Int. J. Environ. Res. Public Health 2020, 17, 4047. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.F.; Jiang, B.; Komal, B.; Bashir, M.A.; Farooq, T.H.; Iqbal, N.; Bashir, M. Correlation between environmental pollution indicators and COVID-19 pandemic: A brief study in Cal-ifornian context. Environ. Res. 2020, 187, 109652. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shi, L.; Zhao, J.; Liu, P.; Sarnat, J.A.; Gao, S.; Schwartz, J.; Liu, Y.; Ebelt, S.T.; Scovronick, N.; et al. Urban Air Pollution May Enhance COVID-19 Case-Fatality and Mortality Rates in the United States. Innovation 2020, 1, 100047. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.R.; DeJoseph, M.E. The Importance of Proper Death Certification during the COVID-19 Pandemic. JAMA 2020, 324, 27. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Thieblemont, C.; Alran, S.; Faivre, S. Impact of the COVID-19 Outbreak on the Management of Patients with Cancer. Target. Oncol. 2020, 15, 249–259. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Rosenbaum, L. The Untold Toll—The Pandemic’s Effects on Patients without Covid-19. N. Engl. J. Med. 2020, 382, 2368–2371. [Google Scholar] [CrossRef]
- Zylke, J.W.; Bauchner, H. Mortality and morbidity: The measure of a pandemic. JAMA 2020, 324, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.S.; Cavalli, L.; Fabrizi, E.; Guastella, G.; Lippo, E.; Parisi, M.L.; Pontarollo, N.; Rizzati, M.; Varacca, A.; Vergalli, S. The Effects of Air Pollution on COVID-19 Related Mortality in Northern Italy. Environ. Resour. Econ. 2020, 76, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Dos Santos, R.S.; Donato, F.D.; De Sario, M.; Michelozzi, P.; Davoli, M.; Masselot, P.; Sera, F.; Gasparrini, A. Excess mortality during the COVID-19 outbreak in Italy: A two-stage interrupted time-series analysis. Int. J. Epidemiol. 2020, 49, 1909–1917. [Google Scholar] [CrossRef]
- Liberti, A. Modern methods for air pollution monitoring. Pure Appl. Chem. 1975, 44, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Powell, H.; Krall, J.R.; Wang, Y.; Bell, M.L.; Peng, R.D. Ambient Coarse Particulate Matter and Hospital Admissions in the Medicare Cohort Air Pollution Study, 1999–2010. Environ. Health Perspect. 2015, 123, 1152–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J. Humidity: Calculate Water Vapor Measures from Temperature and Dew Point. 2019. R package version 0.1.5. Available online: https://github.com/caijun/humidity (accessed on 18 February 2021).
- Armstrong, B.G.; Gasparrini, A.; Tobías, A. Conditional Poisson models: A flexible alternative to conditional logistic case cross-over analysis. BMC Med. Res. Methodol. 2014, 14, 122. [Google Scholar] [CrossRef] [Green Version]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Lian, T.; Fu, Y.; Sun, M.; Yin, M.; Zhang, Y.; Huang, L.; Huang, J.; Xu, Z.; Mao, C.; Ni, J.; et al. Effect of temperature on accidental human mortality: A time-series analysis in Shenzhen, Guangdong Province in China. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Gasparrini, A.; Leone, M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Turner, H.; Firth, D. Generalized Nonlinear Models in R: An Overview of the Gnm Package. 2020. R package version 1.1-1. Available online: https://cran.r-project.org/package=gnm (accessed on 18 February 2021).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Li, S.; Tian, Z.; Pan, X.; Zhang, J.; Williams, G. The burden of air pollution on years of life lost in Beijing, China, 2004–2008: Retrospective regression analysis of daily deaths. BMJ 2013, 347, f7139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Zanobetti, A.; Koutrakis, P.; Schwartz, J.D. Associations of Fine Particulate Matter Species with Mortality in the United States: A Multicity Time-Series Analysis. Environ. Health Perspect. 2014, 122, 837–842. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.Z.S.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Mao, F.; Ding, Z.; Xiang, Q.; Wang, W. Age- and season-specific effects of ambient particles (PM(1), PM(2.5), and PM(10)) on daily emergency de-partment visits among two Chinese metropolitan populations. Chemosphere 2020, 246, 125723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]
- Fattorini, D.; Regoli, F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020, 264, 114732. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Ozgen, C.; Strobl, E. Air Pollution Exposure and Covid-19 in Dutch Municipalities. Environ. Resour. Econ. 2020, 76, 581–610. [Google Scholar] [CrossRef]
- Xiao, W.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Vasquez-Apestegui, V.; Parras-Garrido, E.; Tapia, V.; Paz-Aparicio, V.M.; Rojas, J.P.; Sánchez-Ccoyllo, O.R.; Gonzales, G.F. Association between Air Pollution in Lima and the High Incidence of COVID-19: Findings from a Post Hoc Analysis. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.-J.; Guan, Y.-J. Effect of ambient air pollutants and meteorological variables on COVID-19 incidence. Infect. Control. Hosp. Epidemiol. 2020, 41, 1011–1015. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, J.; Wang, W.; Liu, Z.; Kan, H.; Qiu, Y.; Meng, X.; Wang, W. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total. Environ. 2020, 741, 140396. [Google Scholar] [CrossRef] [PubMed]
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and COVID-19: The Role of Particulate Matter in the Spread and Increase of COVID-19’s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, H.; Chen, J.; Xu, C.; Li, J.; Xu, P.; Sun, Z. Effects of aerosol pollution on PM2.5-associated bacteria in typical inland and coastal cities of northern China during the winter heating season. Environ. Pollut. 2020, 262, 114188. [Google Scholar] [CrossRef]
- Farina, F.; Sancini, G.; Battaglia, C.; Tinaglia, V.; Mantecca, P.; Camatini, M.; Palestini, P. Milano Summer Particulate Matter (PM10) Triggers Lung Inflammation and Extra Pulmonary Adverse Events in Mice. PLoS ONE 2013, 8, e56636. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ma, Z.; Peppelenbosch, M.P.; Pan, Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health 2020, 8, e480. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, L. Facing Covid-19 in Italy—Ethics, Logistics, and Therapeutics on the Epidemic’s Front Line. N. Engl. J. Med. 2020, 382, 1873–1875. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef]
- Barrett, K.; Khan, Y.A.; Mac, S.; Ximenes, R.; Naimark, D.M.; Sander, B. Estimation of COVID-19–induced depletion of hospital resources in Ontario, Canada. Can. Med. Assoc. J. 2020, 192, E640–E646. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabrò, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Hear. J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef]
- Sicard, P.; Khaniabadi, Y.O.; Perez, S.; Gualtieri, M.; De Marco, A. Effect of O3, PM10 and PM2.5 on cardiovascular and respiratory diseases in cities of France, Iran and Italy. Environ. Sci. Pollut. Res. 2019, 26, 32645–32665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, M.; Zhang, J.; Ye, J.; Xu, Y.; Wang, Z.; Ye, D.; Liu, J.; Wan, J. Advances in the relationship between coronavirus infection and cardiovascular diseases. Biomed. Pharmacother. 2020, 127, 110230. [Google Scholar] [CrossRef]
- Kang, S.-J.; Jung, S.I. Age-Related Morbidity and Mortality among Patients with COVID-19. Infect. Chemother. 2020, 52, 154–164. [Google Scholar] [CrossRef]
- Han, F.; Yang, X.; Xu, D.; Wang, Q.; Xu, D. Association between outdoor PM2.5 and prevalence of COPD: A systematic review and meta-analysis. Postgrad. Med. J. 2019, 95, 612–618. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, J.; Ruan, Z.; Yin, P.; Zhang, S.; Liu, J.; Liu, Y.; Li, R.; Wang, L.; Lin, H. Changes in Life Expectancy of Respiratory Diseases from Attaining Daily PM2.5 Standard in China: A Nationwide Observational Study. Innovation 2020, 1, 100064. [Google Scholar] [CrossRef]
- Hoek, G.; Kos, G.; Harrison, R.; de Hartog, J.; Meliefste, K.; ten Brink, H.; Katsouyanni, K.; Karakatsani, A.; Lianou, M.; Kotronarou, A.; et al. Indoor–outdoor relationships of particle number and mass in four European cities. Atmos. Environ. 2008, 42, 156–169. [Google Scholar] [CrossRef]
- Szigeti, T.; Dunster, C.; Cattaneo, A.; Cavallo, D.; Spinazzè, A.; Saraga, D.E.; Sakellaris, I.A.; de Kluizenaar, Y.; Cornelissen, E.J.; Hänninen, O.; et al. Oxidative potential and chemical composition of PM2. 5 in office buildings across Europe—The OFFICAIR study. Environ. Int. 2016, 92–93, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.; Zhao, B. Estimating Mortality Derived from Indoor Exposure to Particles of Outdoor Origin. PLoS ONE 2015, 10, e0124238. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | 2015 | 2016 | 2017 | 2018 | 2019 | 2015–2019 | 2020 | p Value |
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 12.32 (4.51) | 12.20 (4.14) | 13.01 (4.07) | 12.75 (5.18) | 11.48 (3.1) | 12.35 (4.29) | 12.52 (4.40) | <0.001 |
| RH (%) | 67.62 (11.45) | 69.12 (10.43) | 65.64 (10.96) | 72.76 (9.43) | 68.85 (12.07) | 68.80 (11.15) | 65.41 (12.08) | <0.001 |
| PM2.5 (μg/m3) | 15.60 (10.23) | 12.60 (7.71) | 13.59 (9.50) | 13.38 (8.49) | 11.77 (8.02) | 13.55 (9.01) | 12.52 (7.57) | <0.001 |
| PM10 (μg/m3) | 23.05 (12.25) | 20.42 (13.46) | 20.73 (12.45) | 22.66 (12.21) | 19.71 (12.95) | 21.40 (12.7) | 20.54 (13.95) | <0.001 |
| PM2.5–10 (μg/m3) | 8.86 (5.83) | 8.24 (6.78) | 8.46 (5.66) | 8.48 (6.16) | 8.49 (6.07) | 8.51 (6.83) | 8.14 (7.43) | <0.001 |
| NO2 (μg/m3) | 21.54 (11.09) | 19.88 (9.87) | 20.31 (10.84) | 19.36 (10.61) | 17.96 (9.90) | 19.91 (10.56) | 11.96 (7.19) | <0.001 |
| CO (mg/m3) | 0.49 (0.29) | 0.44 (0.25) | 0.41 (0.22) | 0.45 (0.24) | 0.40 (0.17) | 0.44 (0.24) | 0.38 (0.24) | <0.001 |
| O3 (μg/m3) | 92.93 (18.97) | 87.55 (18.94) | 94.95 (18.45) | 85.80 (20.71) | 83.36 (20.75) | 89.15 (20.00) | 85.13 (22.66) | <0.001 |
| SO2 (μg/m3) | 3.35 (2.70) | 2.89 (2.39) | 2.83 (2.46) | 2.63 (2.31) | 2.82 (3.49) | 2.91 (2.66) | 2.46 (2.89) | <0.001 |
| Daily Average deaths counts | ||||||||
| Age < 65 years | 200 (17) | 192 (15) | 190 (15) | 192 (17) | 187(17) | 192 (17) | 196 (37) | 0.097 |
| Age ≥ 65 years | 1590 (150) | 1523 (104) | 1544 (82) | 1548 (154) | 1573 (125) | 1555 (128) | 1939 (556) | <0.001 |
| Female | 941 (93) | 887 (66) | 903 (53) | 906 (93) | 918 (80) | 911 (80) | 1093 (271) | <0.001 |
| Male | 849 (70) | 828 (50) | 831 (46) | 835 (73) | 842 (64) | 837 (62) | 1043 (325) | <0.001 |
| Total | 1790 (157) | 1715 (109) | 1734 (88) | 1740 (161) | 1759 (135) | 1748 (135) | 2136 (590) | <0.001 |
| PM | Subgroups | Attributable Mortality Fractions (%) | Attributable Deaths | ||
|---|---|---|---|---|---|
| 2020 | 2015–2019 | 2020 | 2015–2019 | ||
| PM2.5 | Total | 10.21 (7.13, 13.31) | 2.44 (1.63, 3.23) | 20,062 (13,811, 25,862) | 3927 (2693, 5171) |
| Female | 8.05 (4.15, 11.60) | 3.11 (1.95, 4.22) | 8094 (4129, 11688) | 2605 (1680, 3560) | |
| Male | 12.36 (8.26, 16.29) | 1.70 (0.47, 2.87) | 11,860 (8009, 15371) | 1308 (465, 2182) | |
| Age < 65 years | 7.42 (−0.41, 14.32) | 0.01 (−2.54, 2.43) | 1340 (−86, 2685) | 1 (−474, 454) | |
| Age ≥ 65 years | 10.47 (7.34, 13.76) | 2.73 (1.85, 3.57) | 18,675 (12,494, 23,935) | 3902 (2691, 5006) | |
| PM10 | Total | 7.69 (5.82, 9.59) | 2.49 (1.80, 3.19) | 15,112 (11,381, 18,574) | 3999 (2886, 5111) |
| Female | 7.97 (5.74, 10.09) | 3.16 (2.21, 4.13) | 8013 (5793, 10150) | 2652 (1844, 3449) | |
| Male | 7.38 (4.99, 9.96) | 1.73 (0.72, 2.79) | 7081 (4758, 9233) | 1334 (542, 2103) | |
| Age < 65 years | 5.21 (0.72, 9.78) | 0.3 (−1.87, 2.33) | 942 (147, 1721) | 53 (−345, 418) | |
| Age ≥ 65 years | 7.93 (6.08, 9.85) | 2.75 (2.1, 3.43) | 14,144 (10,859, 17,330) | 3932 (2883, 4948) | |
| PM2.5–10 | Total | 3.66 (2.67, 4.67) | 1.43 (0.88, 1.94) | 7193 (5163, 9144) | 2303 (1494, 3119) |
| Female | 3.88 (2.65, 5.04) | 1.66 (0.99, 2.39) | 3905 (2734, 5073) | 1389 (819, 1991) | |
| Male | 3.43 (2.13, 4.69) | 1.18 (0.39, 1.89) | 3293 (2085, 4452) | 910 (296, 1471) | |
| Age < 65 years | 2.70 (0.15, 4.93) | 0.99 (−0.62, 2.46) | 488 (59, 934) | 175 (−102, 436) | |
| Age ≥ 65 years | 3.76 (2.67, 4.82) | 1.48 (0.97, 1.98) | 6707 (4705, 8451) | 2123 (1375, 2861) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Xu, R.; Yu, W.; Chen, Z.; Guo, Y.; Li, S. Vulnerability and Burden of All-Cause Mortality Associated with Particulate Air Pollution during COVID-19 Pandemic: A Nationwide Observed Study in Italy. Toxics 2021, 9, 56. https://doi.org/10.3390/toxics9030056
Ye T, Xu R, Yu W, Chen Z, Guo Y, Li S. Vulnerability and Burden of All-Cause Mortality Associated with Particulate Air Pollution during COVID-19 Pandemic: A Nationwide Observed Study in Italy. Toxics. 2021; 9(3):56. https://doi.org/10.3390/toxics9030056
Chicago/Turabian StyleYe, Tingting, Rongbin Xu, Wenhua Yu, Zhaoyue Chen, Yuming Guo, and Shanshan Li. 2021. "Vulnerability and Burden of All-Cause Mortality Associated with Particulate Air Pollution during COVID-19 Pandemic: A Nationwide Observed Study in Italy" Toxics 9, no. 3: 56. https://doi.org/10.3390/toxics9030056
APA StyleYe, T., Xu, R., Yu, W., Chen, Z., Guo, Y., & Li, S. (2021). Vulnerability and Burden of All-Cause Mortality Associated with Particulate Air Pollution during COVID-19 Pandemic: A Nationwide Observed Study in Italy. Toxics, 9(3), 56. https://doi.org/10.3390/toxics9030056