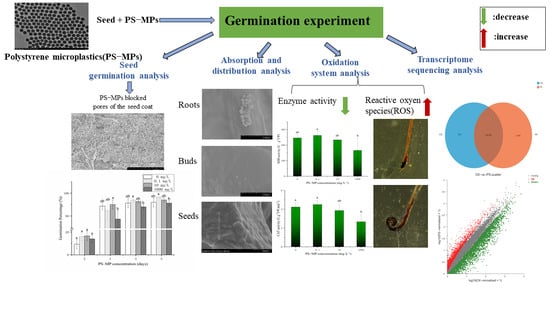
Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Polystyrene Micrometer Plastics
2.2. Seed Soaking, Germination, and Cultivation
2.3. MP Absorption and Distribution Characteristics
2.3.1. Vacuum Freeze-Drying Method for Scanning Electron Microscopy
2.3.2. TEM Embedding
2.4. Determination of Antioxidant Enzyme Activity in Rice Buds
2.5. Transcriptomic Determination of the Root System of Rice Seedlings
2.6. Statistical Analysis
3. Results and Discussion
3.1. Basic Properties of PS-NPs
3.2. Effect of PS-NPs on the Germination of Rice Seeds
3.3. Effects of PS-MPs on the Growth of Rice Roots and Buds
3.4. Absorption and Distribution of PS-MPs in Rice
3.5. Effects of PS-MPs on the Rice Antioxidant System
3.5.1. Effects of PS-MPs on ROS Levels in Rice Roots
3.5.2. Effect of PS-MPs on the Antioxidant Enzyme Activity in Rice Buds
3.6. Transcriptomic Analysis of PS-MPs in Rice Seedling Roots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Peng, G.; Xu, P.; Zhu, B.; Zhu, B.; Bai, M.; Li, D. Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in megacities. Environ. Pollut. 2018, 234, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- MSFD-TSGML. Guidance on Monitoring of Marine Litter in European Seas—A Guidance Document within the Common Implementation Strategy for the Marine Strategy Framework Directive; JRC Scientific and Policy Reports JRC83985; Publications Office of the European Union: Luxembourg, 2013; ISBN 9789279327094.
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crackentry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Bandmann, V.; Müller, J.D.; Kohler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yuan, X.; Jia, Y.; Feng, L.; Zhu, F.; Dong, S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Liao, Y.; Jiahefubek, N.; Li, M.; Wang, X.; Jiang, L. The effect of microplastics on the growth and physiological and biochemical characteristics of wheat. Environ. Sci. 2019, 40, 4661–4667. (In Chinese) [Google Scholar]
- Liu, Y.; Zhang, Q.; Cui, W.; Duan, Z.; Wang, F. Study on the toxicity of polyethylene microplastics on mung bean germination. Environ. Dev. 2019, 31, 123–125. (In Chinese) [Google Scholar]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.A.; Parolini, M. Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Krzan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Zhou, B.S.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619, 1–8. [Google Scholar] [CrossRef]
- Peda, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Kohler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. After an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: A possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef]
- Guan, H.; Han, Z.; Cao, H.; Niu, S.; Qian, Z.; Ye, J.; Ren, L. Characterization of Multi-scale Morphology and Superhydrophobicity of Water Bamboo Leaves and Biomimetic Polydimethylsiloxane (PDMS) Replicas. J. Bionic Eng. 2015, 12, 624–633. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for SEM of plant surfaces. Mater. Today 2010, 12, 32–43. [Google Scholar] [CrossRef]
- Ortúñez, E.; de la Fuente, V. Epidermal micromorphology of the genus Festuca L. (Poaceae) in the Iberian Peninsula. Plant Syst. Evol. 2010, 284, 201–218. [Google Scholar] [CrossRef]
- Helliot, B.; Swennen, R.; Poumay, Y.; Frison, E.; Lepoivre, P.; Panis, B. Ultrastructural changes associated with cryopreservation of banana (Musa spp.) highly proliferating meristems. Plant Cell Rep. 2003, 21, 690–698. [Google Scholar] [CrossRef]
- Xu, P.; Guo, Y.; Bai, J.G.; Shang, L.; Wang, X. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol. Plant 2008, 132, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Guo, Y.; Li, Q.; Zhang, J.; Wang, X.; Bai, J. The pretreatment of cucumber with methyl jasmonate regulates antioxidant enzyme activities and protects chloroplast and mitochondrial ultrastructure in chilling stressed leaves. Sci. Hortic. 2012, 143, 135–143. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Veljovic-Jovanovic, S.; Noctor, G.; Foyer, C.H. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol. Biochem. (Paris) 2002, 40, 501–507. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lin, H.W.; Chern, R.H.; Lo, H.F.; Li, L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul. 1999, 27, 167–172. [Google Scholar] [CrossRef]
- Slooten, L.; Capau, C.; Van Camp, W.; Van Montagu, M.; Sybesma, C.; Inzé, D. Factors affecting enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxyde dismutase in the chloroplast. Plant Physiol. 1995, 107, 737–750. Available online: http://new66.net/s/O0GCa5 (accessed on 21 April 2021). [CrossRef] [Green Version]
- Lulai, E.C.; Neubauer, J.D. Wound-induced suberization genes are differentially expressed, spatially and temporally, during closing layer and wound periderm formation. Postharvest Biol. Technol. 2014, 90, 24–33. [Google Scholar] [CrossRef]
- Pereira, G.; Molina, S.; Lea, P.; Azevedo, R. Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 2002, 239, 123–132. [Google Scholar] [CrossRef]
- Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- Calero, E.; West, S.H.; Hinson, K. Water absorption of soybean seeds and fassociated causal factors. Crop Sci. 1981, 21, 926–933. [Google Scholar] [CrossRef]
- Foolad, M.R.; Lin, G.Y.; Quaslet, C.O. Relationships between coldand salt-tolerance during seed germination in tomato: Germplasm evaluation. Plant Breed. 2008, 118, 45–48. [Google Scholar] [CrossRef]
- Tripathi, S.; Sarkar, S. Influence of water soluble carbon dots on the growth of wheat plant. Appl. Nanosci. 2015, 5, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef]
- Van-Norman, J.M.; Xuan, W.; Beeckman, T.; Benfey, P.N. To branch or not to branch: The role of pre-patterning in lateral root formation. Development 2013, 140, 4301–4310. [Google Scholar] [CrossRef] [Green Version]
- Walch-Liu, P.; Filleur, S.; Gan, Y.B.; Forde, B.G. Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosynth. Res. 2005, 83, 239–250. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kanna, N.S.; Prabu, P. Influence of nanosilica powder on the growth of maize crop (Zea Mays L.). Int. J. Green Nanotechnol. 2011, 3, 180–190. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; De-Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in Agriculture: Which innovation potential does it have. Front. Environ. Sci. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Zhao, Y.; Huang, Y.; Liu, Z. Foliar application with nanosilicon reduced cadmium accumulation in grains by inhibiting cadmium translocation in rice plants. Environ. Sci. Pollut. Res. 2018, 25, 2361–2368. [Google Scholar] [CrossRef]
- Panova, G.G.; Ktitorova, I.N.; Skobeleva, O.V.; Sinjavina, N.G.; Charykov, N.A.; Semenov, K.N. Impact of polyhydroxy fullerene (fullerol or fullerenol) on growth and biophysical characteristics of barley seedlings in favourable and stressful conditions. Plant Growth Regul. 2015, 79, 309–317. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Knight, H.; Knight, M.R. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Wejtaszek, P. Oxidative burst: An early plant response to pathogen infection. Biochem. J. 1997, 332, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Martinez, C.A.; Marcelo, E.L.; Marco, A.O.; Moacyr, M. Differential responses of superoxide dismutase in freezing resistant Solanum curtilobum and freezing sensitive Solanum tuberosum subjected to oxidative and water stress. Plant Sci. 2001, 160, 505–515. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, U.; Wobus, B.; Weschke, W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant. 2000, 109, 435–442. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Lin, C.; Kao, C. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000, 30, 151–155. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Furumoto, K.; Inoue, E.; Nagao, N.; Hiyama, E.; Miwa, N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998, 63, 935–948. [Google Scholar] [CrossRef]
- Treuter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Woolfson, K.N.; Haggitt, M.L.; Zhang, Y.; Kachura, A.; Bjelica, A.; Rincon, M.; Kaberi, K.M.; Bernards, M.A. Differential induction of polar and nonpolar metabolism during wound-induced suberization in potato (Solanum tuberosum L.) tubers. Plant J. Cell Mol. Biol. 2018, 93, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Q.; Liu, Y.; Ye, X.; Jiang, Y.; Zheng, X. Radix Tetrastigma flavonoid ameliorates inflammation and prolongs the lifespan of Caenorhabditis elegans through JNK, p38 and Nrf2 pathways. Free Radic. Res. 2019, 53, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Focke, M.; Pollard, M.; Ohlrogge, J. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J. 2000, 22, 39–50. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Christian, R.H.; Shimizu, T.; Spener, F.; Meer, G.; Michael, J.O.; Edward, A.D. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Fahy, E.; Gupta, S.; Sud, M.; Byrnes, R.W.; Cotter, D.; Dinasarapu, A.R.; Maurya, M.R. Bioinformatics and Systems Biology of the Lipidome. Chem. Rev. 2011, 111, 6452–6490. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhao, M.; Meng, F.; Xiao, Y.; Dai, W.; Luan, Y. Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics 2021, 9, 179. https://doi.org/10.3390/toxics9080179
Zhang Q, Zhao M, Meng F, Xiao Y, Dai W, Luan Y. Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics. 2021; 9(8):179. https://doi.org/10.3390/toxics9080179
Chicago/Turabian StyleZhang, Qiuge, Mengsai Zhao, Fansong Meng, Yongli Xiao, Wei Dai, and Yaning Luan. 2021. "Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity" Toxics 9, no. 8: 179. https://doi.org/10.3390/toxics9080179
APA StyleZhang, Q., Zhao, M., Meng, F., Xiao, Y., Dai, W., & Luan, Y. (2021). Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics, 9(8), 179. https://doi.org/10.3390/toxics9080179