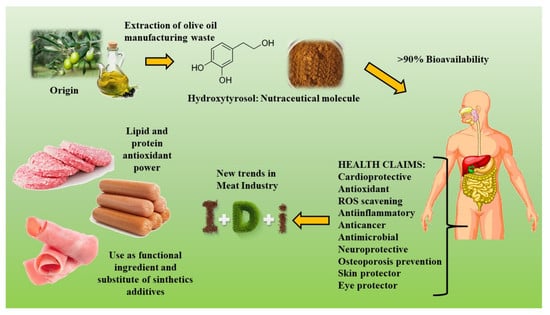
Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat
Abstract
:1. Introduction
2. The Role of HXT in Diet
3. The Role of HXT in Health
4. Preparation of HXT Extract
5. Use of HXT Extract as Functional Ingredient in Meat
6. New Trends
7. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- The International Agency for Research on Cancer (IARC). Q&A on the Carcinogenicity of the Consumption of Red Meat and Processed Meat; Press Release Nº240; Monographs-Q&A; IARC: Geneva, Switzerland, 2015; Volume 114. [Google Scholar]
- Lund, M.N.; Ray, C.A. Control of Maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Soubra, L.; Sarkis, D.; Hilan, C.; Verger, P.H. Dietary exposure of children and teenagers to benzoates, sulphites, butylhydroxyanisol (BHA) and butylhiddroxytoluen (BHT) in Beirut (Lebanon). Regul. Toxicol. Pharmacol. 2007, 47, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Pan, A.Y. Chapter 2: Cumulative Environmental Changes, Skewed Antigen Exposure and the Increase of Allergy. Adv. Inmunol. 2008, 98, 39–83. [Google Scholar]
- Clough, S.R. Sodium Sulfite. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–343. [Google Scholar]
- Nieto, G.; Jongberg, S.; Andersen, M.L.; Skibsted, L.H. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013, 95, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L. Chapter 4: Protein oxidation and implications for muscle food quality. In Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; Decker, E.A., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 85–112. ISBN 0-471-31454-4. [Google Scholar]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, C215–C221. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Shah, M.; Don Bosco, S.J.; Ahmad Mir, S. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): Influence on lamb meat quality. Meat Sci. 2010, 84, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Estrada, M.; Jordán, M.J.; Garrido, M.D.; Bañón, S. Effects in ewe diet of rosemary by-product on lipid oxidation and the eating quality of cooked lamb under retail display conditions. Food Chem. 2011, 124, 1423–1429. [Google Scholar] [CrossRef]
- Lee Richards, K. The Most Powerful Natural Antioxidant Discovered to Date—Hydroxytyrosol. Pro-Health, 2014. Available online: http://www.prohealth.com/library/print.cfm?libid=17054 (accessed on 12 January 2018).
- Yadav, A.S.; Singh, R.P. Natural preservatives in poultry meat products. Nat. Prod. Radiance 2004, 3, 300–303. [Google Scholar]
- Wang, D.; Williams, B.A.; Ferruzzi, M.G.; D’Arcy, B.R. Microbial metabolites, but not other phenolics derived from grape seed phenolic extract, are transported through differentiated Caco-2 cell monolayers. Food Chem. 2013, 138, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Merra, E.; Calzaretti, G.; Bobba, A.; Storelli, M.M.; Casalino, E. Antioxidant role of hydroxytyrosol on oxidative stress in cadmium-intoxicated rats: Different effect in spleen and testes. Drug Chem. Toicol. 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lemonakis, N.; Poudyal, H.; Halabalaki, M.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.L.; Gikas, E. The LC-MS-based metabolomics of hydroxytyrosol administration in rats reveals amelioration of the metabolic syndrome. J. Chromatogr. B 2017, 1041, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Martínez, L.; Castillo, J.; Ros, G. Effect of hydroxytyrosol, walnut and olive oil on nutritional profile of Low-Fat Chicken Frankfurters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600518. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez, L.; Ros, G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000, 9, 869–873. [Google Scholar]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Fanizzi, F.P.; Maffia, M. Composition and statistical analysis of biophenols in Apulian Italian EVOOs. Foods 2017, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Paucar, F.; Tapia, F.; Ortiz, J.; Jimenez, P.; Romero, N. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018, 243, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H. Olive-oil consumption and health: The possible role of antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- MAPAMA (Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente). Informe del consume de alimentación en España. In Taller del Centro de Publicaciones del MAPAMA; MAPAMA: Madrid, Spain, 2016; NIPO 013-17-143-0. [Google Scholar]
- Schaffer, S.; Müller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharm. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Khymenets, O.; Crespo, M.C.; Dangles, O.; Rakotomanomana, N.; Andres-Lacueva, C.; Visioli, F. Human hydroxytyrosol’s absorption and excretion from a nutraceutical. J. Funct. Foods 2016, 23, 278–282. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.G.; López, O.; López-García, M.A.; Marset, A. Chapter 20, Biological properties of Hydroxytyrosol and its derivates. In Olive Oil—Constituents, Quality, Health Properties and Bioconversions; InTech: London, UK, 2012; pp. 375–398. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL-cholesterol concentrations (ID 1639, further assessment) pursuant to Article 13 of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2848. [Google Scholar]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-Rich Olive Mill Waste water Extract Protects Brain Cells in Vitro and ex Vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- López-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodríguez-Pérez, M.D.; Reyes, J.J.; Guzmán-Moscoso, R.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J.; González-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Merola, N.; Castillo, J.; Benavente-García, O.; Ros, G.; Nieto, G. The effect of consumption of citrus fruit and olive leaf extract on lipid metabolism. Nutrients 2017, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Siriani, R.; Chimento, A.; De Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Andò, S.; Maggiolini, M.; et al. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ragione, F.D.; Cucciolla, V.; Borriello, A.; Pietra, V.D.; Pontoni, G.; Racioppi, L.; Manna, C.; Galletti, P.; Zappia, V. Hydroxytyrosol, a natural molecule occurring in olive oil, induces cytochrome c-dependent apoptosis. Biochem. Biophys. Res. Commun. 2000, 278, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Y.Z.; Rowland, I.R.; McGlynn, H.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; Kaisalo, L.; Wähälä, K.; et al. Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. Int. J. Cancer 2008, 122, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Sánchez-Quesada, C.; Campos, C.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, S.; De La Cruz, J.P.; López-Villodres, J.A.; Muñoz-Martín, J.; Guerrero, A.; Reyes, J.J.; Labajos, M.T.; González-Correa, J.A. Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation. J. Nutr. Biochem. 2013, 24, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; De Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Medina, E.; Romero, C.; De Castro, A. Antimicrobial activity of olive oil. Agro Food Ind. Hi-Tech 2007, 18, 6–8. [Google Scholar]
- Furneri, P.M.; Piperno, A.; Sajia, A.; Bisignano, G. Antimycoplasmal activity of hydroxytyrosol. Antimicrob. Agents Chemother. 2004, 48, 4892–4894. [Google Scholar] [CrossRef] [PubMed]
- Yangui, T.; Dhouib, A.; Rhouma, A.; Sayadi, S. Potential of hydroxytyrosol rich composition from olive mill wastewater as a natural disinfectant and its effect on seeds vigour response. Food Chem. 2009, 117, 1–8. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-mollecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol. Biochem. Biophys. Res. Commun. 2007, 354, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Goto, T.; Araki, M.; Miyazaki, H.; Hagiwara, H. Olive polyphenol hydroxytyrosol prevents bone loss. Eur. J. Pharmacol. 2011, 662, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Viola, P.; Viola, M. Virgin olive oil as a fundamental nutritional component and skin protector. Clin. Dermatol. 2009, 27, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Consejo Superior De Investigaciones Científicas (Csic). Method for Obtaining Hydroxytyrosol Extract, Mixture of Hydroxytyrosol and 3,4-dihydroxyphenylglycol Extract, and Hydroxytyrosyl Acetate Extract, from By-Products of the Olive Tree, and the Purification Thereof; WO2013007850 A1; Universidad De Sevilla: Seville, Spain, 2013. [Google Scholar]
- AECOSAN (Agencia Española de Consumo, Seguridad Alimentaria y Nutrición) (Food Safety and Nutrition Section of the Scientific Committee). Report of the Scientific Committee of the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN) on a request for initial assessment for marketing of synthetic hydroxytyrosol under Regulation (EC) No 258/97 concerning novel foods and novel food ingredients. Rev. Com. Cient. AECOSAN 2015, 21, 11–25. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728. [Google Scholar]
- Rounds, L.; Havens, C.M.; Feinstein, Y.; Friedman, M.; Ravishankar, S. Concentration dependent inhibition of Escherichia coli O157:H7 and heterocyclic amines in heated ground beef patties by apple and olive extracts, onion powder and clove bud oil. Meat Sci. 2013, 94, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Santos-López, J.A.; Freire, M.; Benedí, J.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. LWT Food Sci. Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef]
- Chaves-Lopez, C.; Serio, A.; Mazzarrino, G.; Martuscelli, M.; Scarpone, E.; Paparella, A. Control of household mycoflora in fermented sausages using phenolic fractions from olive mill wastewaters. Int. J. Food Microbiol. 2015, 207, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Muíño, I.; Díaz, M.T.; Apeleo, E.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzurica, S.; De la Fuente, J. Valorisation of an extract from olive oil waste as a natural antioxidant for reducing meat waste resulting from oxidative processed. J. Clean. Product. 2016, 140, 924–932. [Google Scholar] [CrossRef]
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Berasategi-Zabalza, I.; Rodríguez-Estrada, M.T.; Novelli, E.; Fasolato, L. Effect of phenols extracted from a by-product of the oil mill on the shelf-life of raw and cooked fresh pork sausages in the absence of chemical additives. LWT Food Sci. Technol. 2017, 85, 89–95. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalaza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative status and presence of bioactive compounds in meat from chickens fed polyphenols extracted from olive oil industry waste. Sustainability 2017, 9. [Google Scholar] [CrossRef]
- Freire, M.; Bou, R.; Cofrades, S.; Jiménez-Colmenero, F. Technological characteristics of cold-set gelled double emulsion enriched with n-3 fatty acids: Effect of hydroxytyrosol addition and chilling storage. Food Res. Int. 2017, 100, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Moudache, M.; Nerín, C.; Colon, M.; Zaidi, F. Antioxidant effect of an innovative active plastic film containing olive leaves extract on fresh pork meat and its evaluation by Raman spectroscopy. Food Chem. 2017, 2291, 98–103. [Google Scholar] [CrossRef] [PubMed]


| Extract Form | Concentration (ppm) | Meat Product | Test Setup | Tested Parameters | Results | Reference |
|---|---|---|---|---|---|---|
| Commercial | 10,000 30,000 | Ground beef patties | Ground beef was mixed with extract and inoculated with E. coli (107 cfu/g). Uniform patties were formed, cooked and shock-cooled. | Total viable count | It was enhanced cell count reduction (3% survivors detectable) | [53] |
| Amine quantification | Reduction of amine formation (50.6%) | |||||
| Commercial | 100 | Pork meat with W1/O/W2 emulsions | Ground pork meat and fat were mixed with W1/O/W2 emulsions and chia oil. Emulsions were vacuum packaged and keep in chilled storage (4 °C) until analyses on the 1st, 7th, 19th, 28th and 39th days. | Light microscopy | Particle size in samples with HXT was higher (p < 0.05) | [54] |
| Antioxidant activity: DPPH | Chia oil presence in meat samples increased oxidation, however, HXT acted as antioxidant (8%). | |||||
| Lipid oxidation (TBARs) | HXT presence in meat samples reduced lipid oxidation by more than 50%. | |||||
| Concentrate | 100.23 | Fermented sausages | During the drying process fermented sausages were dipped in extract solutions (2.5–5%) for 1 min at 20 °C and were continued drying. | Total viable count | No differences | [55] |
| Lactic acid bacteria | No differences | |||||
| Micrococci | Growth reduction affected volatile compound profile | |||||
| Yeast | No significant differences | |||||
| Moulds | Reduction of species | |||||
| pH | No significant differences | |||||
| Water activity | No significant differences | |||||
| Lipid oxidation (TBARs) | Reduced values (12–38%) | |||||
| Volatile compounds | Reduces volatile compounds from microbial esterification and lipid oxidation | |||||
| Sensory attributes (colour) | Redness increased | |||||
| Commercial | 100, 200, 400 | Lamb meat patties | Minced lamb meat enriched in omega-3 fatty acids (with fish oil) was mixed with natural extracts and stored in high-oxygen modified atmosphere packs for up to 9 days at 4 °C. | In vitro antioxidant activity (ORAC and FRAP) | ORAC: No significant differences | [56] |
| FRAP: antioxidant activity increases with extract presence | ||||||
| Colour (CIELab) | Lightness (L*) increased in samples without extract by changes in muscle proteins. | |||||
| Significant differences between samples at day 3, 6 and 9 of storage. | ||||||
| Lipid oxidation (TBARs) | No significant differences | |||||
| Protein oxidation (thiol and carbonyl groups) | Natural extracts improvement texture but it alteration odour and flavour. | |||||
| Sensory analysis | ||||||
| Concentrated, undefined | 75, 150 | Pork sausages | Ground pork (50/50 - meat/fat) was minced and mixed with salt and phenols. Mix was stuffed into 40-mm diameter bovine casings, were left to drip at 15 °C for 6 h and stored without packaging alternating fluorescent light (12 h dark and 12 h light) at 2 °C for 14 days. After, sausages were cooked, stored 72 h at 4 °C and frozen until analysis (80 °C) | Nutritional composition | No differences | [57] |
| pH | No differences | |||||
| Cooking loss | No differences | |||||
| Diacylglycerols | Phenols had an inhibitory effect on microorganisms and a reduction in lipolytic activity. | |||||
| Lipid oxidation (TBARs) | Oxidation was reduced (>40%) as TBARs as POV. But there are no differences in COPs. | |||||
| Peroxide value (POV) | ||||||
| Cholesterol oxidation products (COPs) | ||||||
| Sensory analysis | Phenols presence was valorated worst by panellists. | |||||
| Commercial 1. HXT 23% from olive waters 2. HXT 7% from olive leaves 3. HXT 7% from olive waters | 50 | Chicken sausages | Pork fat and chicken meat were minced and mixed with walnuts, EVOO and three HXT extracts. Samples were cooked for 3 h at 72 °C, packaged in MAP (10% O2/20% CO2/10% N2) and stored at 4 °C for 21 days. | Nutritional composition | No differences | [21] |
| Colour (CIELab) | L* and b* were lower in samples with HXT and EVOO, while a* was higher | |||||
| Cooking loss | Cooking loss values were higher in samples with HXT | |||||
| Lipid oxidation (TBARs) | In samples with HXT TBARs value was lower and in samples with HXT and EVOO, lipid oxidation was 71% lower than control. | |||||
| Protein oxidation (thiol groups) | HXT reduced protein oxidation between 13–25%. | |||||
| Scanning electron microscopy | Sausages incorporated HXT showed different structures. | |||||
| Sensory analysis | Samples with HXT 7% from olive water was accepted, while other samples with HXT presented lowest acceptability. | |||||
| Commercial, 7% from olive waters | 50 | Chicken Frankfurters | Back fat and chicken meat were minced and mixed with walnuts, EVOO and HXT. Samples were cooked for 3 h at 72 °C, packaged in MAP (10% O2/20% CO2/10% N2) and stored at 4 °C for 21 days. | Nutritional composition | No differences | [20] |
| Mineral content | Ca, K, Fe, Mg, P, Mn and Zn concentrations were higher in samples with HXT. | |||||
| No differences | ||||||
| Fatty acid profile. Sensory analysis | Extracted flavour and odour parameters were increased in samples with HXT but it was accepted by panellists. | |||||
| Olive cake applied in chicken feed | 4.6 9.5 | Chicken meat | 297 chickens were feeding until 21 days of age with three treatments: basal diet, diet supplemented with 82.5 g/kg olive phenols and diet supplemented with 165 g/kg olive phenols. Chickens were weighed at 28, 35 and 42 days of age and slaughtered at 42th. Carcasses were maintained at −20 °C for three months until consumption and at −80 °C for other analyses. | Chicken weight | The chicken weight was higher | [58] |
| Colour (CIELab) | L* and b* was higher while a* was lower | |||||
| Cooking loss | No differences | |||||
| Nutritional composition | No differences | |||||
| pH | No differences | |||||
| Lipid oxidation (TBARs) | Lipid oxidation was lower | |||||
| Antioxidant capacity (DPPH) | Samples with HXT showed a high antioxidant capacity | |||||
| Sensory analysis | No differences, so HXT did not alter the sensory quality. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. https://doi.org/10.3390/medicines5010013
Martínez L, Ros G, Nieto G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines. 2018; 5(1):13. https://doi.org/10.3390/medicines5010013
Chicago/Turabian StyleMartínez, Lorena, Gaspar Ros, and Gema Nieto. 2018. "Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat" Medicines 5, no. 1: 13. https://doi.org/10.3390/medicines5010013
APA StyleMartínez, L., Ros, G., & Nieto, G. (2018). Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines, 5(1), 13. https://doi.org/10.3390/medicines5010013