Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications
Abstract
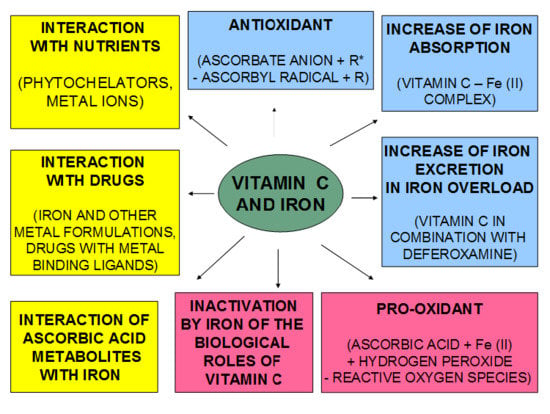
:1. Introduction
2. Historical Perspective of Ascorbic Acid
3. The Biological Redox and Toxic Effects of Iron
4. Iron Coordination and Redox Chemistry of Ascorbic Acid
5. Biological Implications of the Iron Complexes of Ascorbic Acid
6. Toxicity Implications of the Interaction of Iron and Ascorbate in Physiological and Iron Loaded Conditions
7. Nutritional and Vitamin C Functional Implications of the Interactions with Iron
8. Pharmacological Characteristics of Ascorbic Acid and Implications for Iron Metabolism
9. The Role of Ascorbic Acid in Iron Absorption and Iron Excretion
10. Drug Interactions with Ascorbic Acid and Iron
11. Future Therapeutic Strategies and Health Implications of the Use of Vitamin C and Iron
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A Precious Metal: Iron, an Essential Nutrient for all Cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoghiorghes, G.; Kolnagou, A. Molecular Factors and Mechanisms Affecting Iron and Other Metal Excretion or Absorption in Health and Disease. The Role of Natural and Synthetic Chelators. Curr. Med. Chem. 2005, 12, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1559S–1566S. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Dietary and Pharmacological Factors Affecting Iron Absorption in Mice and Man (Comment for a Letter to the Editor). Haematologica 2016, 101, 120–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide Prevalence of Anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Community Control of Hereditary Anaemias: Memorandum from a WHO Meeting. Bull. World Health Organ. 1983, 61, 63–80. [Google Scholar]
- Weatherall, D.J.; Clegg, J.B. Inherited haemoglobin disorders: An Increasing Global Health Problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar] [CrossRef]
- Zurlo, M.G.; De Stefano, P.; Borgna-Pignatti, C.; Di Palma, A.; Melevendi, C.; Piga, A.; Di Gregorio, F.; Burattini, M.G.; Terzoli, S. Survival and Causes of Death in Thalassaemia Major. Lancet 1989, 2, 27–30. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. How to Manage Iron Toxicity in Post-Allogeneic Hematopoietic Stem Cell Transplantation? Expert Rev. Hematol. 2020, 13, 299–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzsimons, E.J.; Cullis, J.O.; Thomas, D.W.; Tsochatzis, E.; Griffiths, W.J.H. Diagnosis and Therapy of Genetic Haemochromatosis (Review and 2017 Update). Br. J. Haematol. 2018, 181, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Felitti, V.J.; Koziol, J.A.; Ho, N.J.; Gelbart, T. Penetrance of 845G → A (C282Y) HFE Hereditary Haemochromatosis Mutation in the USA. Lancet 2002, 359, 211–218. [Google Scholar] [CrossRef]
- Andrews, N.C. Forging a Field: The Golden Age of Iron Biology. Blood 2008, 112, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Editorial: Free Radicals and Antioxidant Protection: Mechanisms and Significance in Toxicology and Disease. Hum. Exp. Toxicol. 1988, 7, 7–13. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative Stress and Iron Homeostasis: Mechanistic and Health Aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanasʹev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 9780824753566. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.; Cross, C.E. Free Radicals, Antioxidants, and Human Disease: Where Are We Now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar]
- Kontoghiorghes, G.J. Iron chelation in biochemistry and medicine. In Free radicals, Oxidant Stress and Drug Action; Rice-Evans, C., Ed.; Rechelieu Press: London, UK, 1987; pp. 277–303. [Google Scholar]
- Kontoghiorghes, G.J.; Jackson, M.J.; Lunec, J. In Vitro Screening of Iron Chelators Using Models of Free Radical Damage. Free Radic. Res. Commun. 1986, 2, 115–124. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jo, E.J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.K.; Lee, M.K.; Lee, K. Combined Vitamin C, Hydrocortisone, and Thiamine Therapy for Patients with Severe Pneumonia Who Were Admitted to the Intensive Care Unit: Propensity Score-Based Analysis of a Before-After Cohort Study. J. Crit. Care 2018, 47, 211–218. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and Vitamin C Deficiency in Critically Ill Patients Despite Recommended Enteral and Parenteral Intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Berenson, J.R.; Matous, J.; Swift, R.A.; Mapes, R.; Morrison, B.; Yeh, H.S. A Phase I/II Study of Arsenic Trioxide/Bortezomib/Ascorbic Acid Combination Therapy for the Treatment of Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2007, 13, 1762–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbarayan, P.R.; Lima, M.; Ardalan, B. Arsenic Trioxide/Ascorbic Acid Therapy in Patients with Refractory Metastatic Colorectal Carcinoma: A Clinical Experience. Acta Oncol. (Madr.) 2007, 46, 557–561. [Google Scholar] [CrossRef]
- Bael, T.E.; Peterson, B.L.; Gollob, J.A. Phase II Trial of Arsenic Trioxide and Ascorbic Acid with Temozolomide in Patients with Metastatic Melanoma with or Without Central Nervous System Metastases. Melanoma Res. 2008, 18, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Fillenbaum, G.G.; Kuchibhatla, M.N.; Hanlon, J.T.; Artz, M.B.; Pieper, C.F.; Schmader, K.E.; Dysken, M.W.; Gray, S.L. Dementia and Alzheimer’s Disease in Community-Dwelling Elders Taking Vitamin C and/or Vitamin E. Ann. Pharmacother. 2005, 39, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Keaney, J.F.; Frei, B.; Holbrook, M.; Olesiak, M.; Zachariah, B.J.; Leeuwenburgh, C.; Heinecke, J.W.; Vita, J.A. Long-Term Ascorbic Acid Administration Reverses Endothelial Vasomotor Dysfunction in Patients with Coronary Artery Disease. Circulation 1999, 99, 3234–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Karne, R.J.; Hall, G.; Campia, U.; Panza, J.A.; Cannon, R.O.; Wang, Y.; Katz, A.; Levine, M.; Quon, M.J. High-Dose Oral Vitamin C Partially Replenishes Vitamin C Levels in Patients with Type 2 Diabetes and Low Vitamin C Levels but Does Not Improve Endothelial Dysfunction or Insulin Resistance. Am. J. Physiol. Hear. Circ. Physiol. 2006, 290, H137–H145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C. A New Clinical Trial to Test High-Dose Vitamin C in Patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef] [Green Version]
- Bors, W.; Buettner, G.R. The vitamin C radical and its reactions. In Vitamin C in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 75–94. [Google Scholar]
- Zümreoglu-Karan, B. The Coordination Chemistry of Vitamin C: An Overview. Coord. Chem. Rev. 2006, 250, 2295–2307. [Google Scholar] [CrossRef]
- Macan, A.M.; Kraljević, T.G.; Raić-malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Naidu, K.A. Vitamin C in Human Health and Disease Is Still a Mystery? An Overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K. Studies on Free Radicals, Antioxidants, and Co-Factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef] [Green Version]
- Bielski, B.H.J.; Allen, A.O.; Schwarz, H.A. Mechanism of Disproportionation of Ascorbate Radicals. J. Am. Chem. Soc. 1981, 103, 3516–3518. [Google Scholar] [CrossRef]
- Bielski, B.H.J. Chemistry of Ascorbic Acid Radicals. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; Advances in Chemistry: Washington, WA, USA, 1982; pp. 81–100. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. The Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Hider, R.H. Iron and Redox Cycling. Do’s and Don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef]
- Erdem, G.; Öner, C.; Önal, A.M.; Kisakürek, D.; Ögüs, A.Y. Free Radical Mediated Interaction of Ascorbic Acid and Ascorbate/Cu(II) with Viral and Plasmid DNAs. J. Biosci. 1994, 19, 9–17. [Google Scholar] [CrossRef]
- Gerster, H. High-Dose Vitamin C: A Risk for Persons with High Iron Stores? Int. J. Vitam. Nutr. Res. 1999, 69, 67–82. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C Act as a Pro-Oxidant under Physiological Conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, P. Mega-Dose Vitamin C as Therapy for Human Cancer? Proc. Natl. Acad. Sci. USA 2008, 105, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittes, R.E. Vitamin C and Cancer. N. Engl. J. Med. 1985, 312, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H. Phase I Clinical Trial of i.v. Ascorbic Acid in Advanced Malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Hoffer, L.J.; Levine, M. Intravenously Administered Vitamin C as Cancer Therapy: Three Cases. CMAJ 2006, 174, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Assouline, S.; Miller, W.H. High-Dose Vitamin C Therapy: Renewed Hope or False Promise? CMAJ 2006, 174, 956–957. [Google Scholar] [CrossRef] [Green Version]
- Frei, B.; Lawson, S. Vitamin C and Cancer Revisited. Proc. Natl. Acad. Sci. USA 2008, 105, 11037–11038. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic Doses of Ascorbate Act as a Prooxidant and Decrease Growth of Aggressive Tumor Xenografts in Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [Green Version]
- Rottenberg, S.; Jonkers, J. Modeling Therapy Resistance in Genetically Engineered Mouse Cancer Models. Drug Resist. Updat. 2008, 11, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Verrax, J.; Cadrobbi, J.; Marques, C.; Taper, H.; Habraken, Y.; Piette, J.; Calderon, P.B. Ascorbate Potentiates the Cytotoxicity of Menadione Leading to an Oxidative Stress That Kills Cancer Cells by a Non-Apoptotic Caspase-3 Independent form of Cell Death. Apoptosis 2004, 9, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Verrax, J.; Stockis, J.; Tison, A.; Taper, H.S.; Calderon, P.B. Oxidative Stress by Ascorbate/Menadione Association Kills K562 Human Chronic Myelogenous Leukaemia Cells and Inhibits Its Tumour Growth in Nude Mice. Biochem. Pharmacol. 2006, 72, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. Supplemental Ascorbate in the Supportive Treatment of Cancer: Reevaluation of Prolongation of Survival Times in Terminal Human Cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomew, M. James Lind’s Treatise of the Scurvy (1753). Postgrad. Med. J. 2002, 78, 695–696. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Ernst, E. Trick or Treatment: The Undeniable Facts about Alternative Medicine; Bantam Press: New York, NY, USA, 2008; ISBN 0-593-06129-2. [Google Scholar]
- Hvoslef, J. The crystal structure of L-ascorbic acid, “vitamin C”. The x-ray analysis. Acta Crystallogr. B 1968, 24, 23–25. [Google Scholar] [CrossRef]
- Hvoslef, J. The Crystal Structure of L-Ascorbic Acid, “Vitamin C”. II. The Neutron Diffraction Analysis. Acta Crystallogr. B 1968, 24, 1434–1440. [Google Scholar] [CrossRef]
- Martell, A.E. Ascorbic Acid: Chemistry, Metabolism, and Uses. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; American Chemical Society: Columbia, WA, USA, 1982; ISBN 780841206328. [Google Scholar]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Smirnoff, N. Ascorbic Acid: Metabolism and Functions of a Multi-Facetted Molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Badu-Boateng, C.; Naftalin, R.J. Ascorbate and Ferritin Interactions: Consequences for Iron Release In Vitro and In Vivo and Implications for Inflammation. Free Radic. Biol. Med. 2019, 133, 75–87. [Google Scholar] [CrossRef]
- La, A.; Nguyen, T.; Tran, K.; Sauble, E.; Tu, D.; Gonzalez, A.; Kidane, T.Z.; Soriano, C.; Morgan, J.; Doan, M.; et al. Mobilization of Iron from Ferritin: New Steps and Details. Metallomics 2018, 10, 154–168. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Prospects for the Introduction of Targeted Antioxidant Drugs for the Prevention and Treatment of Diseases Related to Free Radical Pathology. Expert Opin. Investig. Drugs 2019, 28, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Stefani, C.; Toyokuni, S.; Ganz, T.; Anderson, G.J.; Subramaniam, N.V.; Trinder, D.; Olynyk, J.K.; Chua, A.; Jansson, P.J.; et al. Redox Cycling Metals: Pedaling Their Roles in Metabolism and Their Use in the Development of Novel Therapeutics. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 727–748. [Google Scholar] [CrossRef]
- Breuer, W.; Hershko, C.; Cabantchik, Z.I. The Importance of Non-Transferrin Bound Iron in Disorders of Iron Metabolism. Transfus. Sci. 2000, 23, 185–192. [Google Scholar] [CrossRef]
- Hahn, P.; Milam, A.H.; Dunaief, J.L. Maculas Affected by Age-Related Macular Degeneration Contain Increased Chelatable Iron in the Retinal Pigment Epithelium and Bruch’s Membrane. Arch. Ophthalmol. 2003, 121, 1099–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron Accumulation in Alzheimer Disease is a Source of Redox-Generated Free Radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Jiang, H.; Xu, H.; Song, N.; Xie, J. Increased Iron Levels Correlate with the Selective Nigral Dopaminergic Neuron Degeneration in Parkinson’s Disease. J. Neural Transm. 2011, 118, 361–369. [Google Scholar] [CrossRef]
- Richardson, D.R. Iron Chelators as Therapeutic Agents for the Treatment of Cancer. Crit. Rev. Oncol. Hematol. 2002, 42, 267–281. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Richardson, D.R. Future of Toxicology—Iron Chelators and Differing Modes of Action and Toxicity: The Changing Face of Iron Chelation Therapy. Chem. Res. Toxicol. 2007, 20, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosato, M.; Di Marco, V. Metal Chelation Therapy and Parkinson’s Disease: A Critical Review on the Thermodynamics of Complex Formation between Relevant Metal Ions and Promising or Established Drugs. Biomolecules 2019, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Heffeter, P.; Pape, V.F.S.; Enyedy, É.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxidants Redox Signal 2019, 30, 1062–1082. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik, N.; Chmielniak, U. Studies on the Redox Activity of Iron N,O-Complexes: Potential T1-Contrast Agents. Redox Rep. 2016, 21, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, N.; Chakraborty, A.; Singh, U.P.; Roy, P.; Ghosh, K. Mononuclear iron(III) Complexes of Tridentate Ligands with Efficient Nuclease Activity and Studies of Their Cytotoxicity. Org. Biomol. Chem. 2015, 13, 11445–11458. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Inhibition of Fe2+- and Fe3+-Induced Hydroxyl Radical Production by the Iron-Chelating Drug Deferiprone. Free Radic. Biol. Med. 2015, 78, 118–122. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Effective Inhibition of Copper-Catalyzed Production of Hydroxyl Radicals by Deferiprone. J. Biol. Inorg. Chem. 2019, 24, 331–341. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.; Eracleous, E.; Economides, C.; Kolnagou, A. Advances in Iron Overload Therapies. Prospects for Effective Use of Deferiprone (L1), Deferoxamine, the New Experimental Chelators ICL670, GT56-252, L1NAll and Their Combinations. Curr. Med. Chem. 2005, 12, 2663–2681. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Clinical Use, Therapeutic Aspects and Future Potential of Deferiprone in Thalassemia and Other Conditions of Iron and Other Metal Toxicity. Drugs Today (Barc.) 2001, 37, 23–35. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Prospects for Introducing Deferiprone as Potent Pharmaceutical Antioxidant. Front. Biosci. (Elite Ed.) 2009, 1, 161–178. [Google Scholar]
- Keypour, H.; Silver, J.; Wilson, M.T.; Hamed, M.Y. Studies on the Reactions of Ferric Iron with Ascorbic Acid. A Study of Solution Chemistry Using Mössbauer Spectroscopy and Stopped-Flow Techniques. Inorg. Chim. Acta 1986, 125, 97–106. [Google Scholar] [CrossRef]
- Grillet, L.; Ouerdane, L.; Flis, P.; Hoang, M.T.T.; Isaure, M.P.; Lobinski, R.; Curie, C.; Mari, S. Ascorbate Efflux as a New Strategy for Iron Reduction and Transport in Plants. J. Biol. Chem. 2014, 289, 2515–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.; Dash, A.C. Phenol-Amide Chelates of Iron(III). Kinetics and Mechanism of Reversible Formation of (Diaqua) (1,3) Bis (2-Hydroxybenzamido)Propaneiron(III) and Its Reactions with Thiocyanate, Azide, Imidazole, Sulphur(IV) and Ascorbic Acid in Aqueous Medium. Indian J. Chem. Sect. Inorg. Phys. Theor. Anal. Chem. 2003, 42, 2427–2438. [Google Scholar]
- Rath, H.; Pradhan, G.C.; Dash, A.C. Phenol-Amide Chelate of iron(III): Its Redox Activity with L-Ascorbic Acid. Indian J. Chem. Sect. Inorg. Phys. Theor. Anal. Chem. 2001, 40, 437–441. [Google Scholar]
- Ortega-Castro, J.; Frau, J.; Casasnovas, R.; Fernández, D.; Donoso, J.; Muñoz, F. High- and Low-Spin Fe(III) Complexes of Various AGE Inhibitors. J. Phys. Chem. A 2012, 116, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Hininger, I.; Waters, R.; Osman, M.; Garrel, C.; Fernholz, K.; Roussel, A.M.; Anderson, R.A. Acute Prooxidant Effects of Vitamin C in EDTA Chelation Therapy and long-Term Antioxidant Benefits of Therapy. Free Radic. Biol. Med. 2005, 38, 1565–1570. [Google Scholar] [CrossRef]
- Martinez, P.; Uribe, D. Study of the Complexes of the Ascorbic Acid-Iron(III) System. Z. Naturforsch. Sect. B J. Chem. Sci. 1982, 37, 1446–1449. [Google Scholar] [CrossRef]
- Jabs, W.; Kruger, G.; Trautwein, A.D. Biomimetic Model Compounds of Metalloproteins and Metalloenzymes: 4. The Complex Formation in the NO. In Bioinorg Chemistry: Transition Metals in Biology and Their Coordination Chemistry: Research Report; Trautwein, A., Ed.; Wiley-VCH: Weinheim, German, 1997; p. 468. [Google Scholar]
- Rao, C.P.; Geetha, K.; Raghavan, M.S.S.; Sreedhara, A.; Tokunaga, K.; Yamaguchi, T.; Jadhav, V.; Ganesh, K.N.; Krishnamoorthy, T.; Ramaiah, K.V.A.; et al. Transition Metal Saccharide Chemistry and Biology: Syntheses, Characterization, Solution Stability and Putative Bio-Relevant Studies of Iron-Saccharide Complexes. Inorg. Chim. Acta 2000, 297, 373–382. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic Acid/Fe@Fe2O3: A Highly Efficient Combined Fenton Reagent to Remove Organic Contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [CrossRef] [Green Version]
- He, D.Q.; Zhang, Y.J.; Pei, D.N.; Huang, G.X.; Liu, C.; Li, J.; Yu, H.Q. Degradation of Benzoic Acid in an Advanced Oxidation Process: The Effects of reducing Agents. J. Hazard. Mater. 2020, 382, 121090. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Kispert, L.D. Carotenoids as Scavengers of Free Radicals in a Fenton Reaction: Antioxidants or Pro-Oxidants? Free Radic. Biol. Med. 2001, 31, 398–404. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Konovalova, T.A.; Kispert, L.D. Antioxidant and Redox Properties of Supramolecular Complexes of Carotenoids with β-Glycyrrhizic Acid. Free Radic. Biol. Med. 2006, 40, 1804–1809. [Google Scholar] [CrossRef]
- Koppenol, W.H. Thermodynamics of Fenton-driven Haber-Weiss and related reactions. In Oxy Radicals and Their Scavenging Systems; Cohen, G., Greenwald, R.A., Eds.; Molecular Aspects, Elsevier Biomedical: New York, NY, USA, 1983; pp. 84–88. [Google Scholar]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode Potentials of Partially Reduced Oxygen Species, from Dioxygen to Water. Free Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P. Free Manganese(II) and Iron(II) Cations Can Act as Intracellular Cell Controls. FEBS Lett. 1982, 140, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Adam, F.I.; Bounds, P.L.; Kissner, R.; Koppenol, W.H. Redox Properties and Activity of Iron-Citrate Complexes: Evidence for Redox Cycling. Chem. Res. Toxicol. 2015, 28, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Merkofer, M.; Kissner, R.; Hider, R.C.; Koppenol, W.H. Redox Properties of the Iron Complexes of Orally Active Iron Chelators CP20, CP502, CP509, and ICL670. Helv. Chim. Acta 2004, 87, 3021–3034. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Antioxidant Targeting by Deferiprone in Diseases Related to Oxidative Damage. Front. Biosci. 2014, 19, 862–885. [Google Scholar] [CrossRef] [Green Version]
- Margolis, S.A.; Duewer, D.L. Measurement of Ascorbic Acid in Human Plasma and Serum: Stability, Intralaboratory Repeatability, and Interlaboratory Reproducibility. Clin. Chem. 1996, 42, 1257. [Google Scholar] [CrossRef] [Green Version]
- Merkofer, M.; Kissner, R.; Hider, R.C.; Brunk, U.T.; Koppenol, W.H. Fenton Chemistry and Iron Chelation under Physiologically Relevant Conditions: Electrochemistry and Kinetics. Chem. Res. Toxicol. 2006, 19, 1263–1269. [Google Scholar] [CrossRef]
- Muneta, P.; Kaisaki, F. Ascorbic Acid-Ferrous Iron (Fe++)Complexes and after Cooking Darkening of Potatoes. Am. Potato J. 1985, 62, 531–536. [Google Scholar] [CrossRef]
- Scheers, N.; Andlid, T.; Alminger, M.; Sandberg, A.S. Determination of Fe2+ and Fe3+ in Aqueous Solutions Containing Food Chelators by Differential Pulse Anodic Stripping Voltammetry. Electroanalysis 2010, 22, 1090–1096. [Google Scholar] [CrossRef]
- Thumser, A.E.; Rashed, A.A.; Sharp, P.A.; Lodge, J.K. Ascorbate Enhances Iron Uptake into Intestinal Cells through Formation of a FeCl3-ascorbate Complex. Food Chem. 2010, 123, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.J.R.; Robinson, S.R.; Czerwinska, H.; Bishop, G.M.; Lawen, A. Two Routes of Iron Accumulation in Astrocytes: Ascorbate-Dependent Ferrous Iron Uptake via the Divalent Metal Transporter (DMT1) Plus an Independent Route for Ferric Iron. Biochem. J. 2010, 432, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoghiorghes, G.J. Structure/red blood cell permeability. Activity of Iron(III) Chelator Complexes. Inorg. Chim. Acta 1988, 151, 101–106. [Google Scholar] [CrossRef]
- Forsbeck, K.; Nilsson, K.; Kontoghiorghes, G.J. Variation in Iron Accumulation, Transferrin Membrane Binding and DNA Synthesis in the K-562 and U-937 Cell Lines Induced by Chelators and Their Iron Complexes. Eur. J. Haematol. 1987, 39, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.J.; Hawkins, C.L.; Lovejoy, D.B.; Richardson, D.R. The Iron Complex of Dp44mT Is Redox-Active and Induces Hydroxyl Radical Formation: An EPR Study. J. Inorg. Biochem. 2010, 104, 1224–1228. [Google Scholar] [CrossRef]
- Escolar, E.; Lamas, G.A.; Mark, D.B.; Boineau, R.; Goertz, C.; Rosenberg, Y.; Nahin, R.L.; Ouyang, P.; Rozema, T.; Magaziner, A.; et al. The Effect of an EDTA-Based Chelation Regimen on Patients with Diabetes Mellitus and Prior Myocardial Infarction in the Trial to Assess Chelation Therapy (TACT). Circ. Cardiovasc. Qual. Outcomes 2014, 7, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Lamas, G.A.; Boineau, R.; Goertz, C.; Mark, D.B.; Rosenberg, Y.; Stylianou, M.; Rozema, T.; Nahin, R.L.; Terry Chappell, L.; Lindblad, L.; et al. EDTA Chelation Therapy Alone and in Combination with Oral High-Dose Multivitamins and Minerals for Coronary Disease: The Factorial Group Results of the Trial to Assess Chelation Therapy. Am. Heart J. 2014, 168, 37–44. [Google Scholar] [CrossRef]
- Born, T.; Kontoghiorghe, C.N.; Spyrou, A.; Kolnagou, A.; Kontoghiorghes, G.J. EDTA Chelation Reappraisal Following New Clinical Trials and Regular Use in Millions of Patients: Review of Preliminary Findings and Risk/Benefit Assessment. Toxicol. Mech. Methods 2013, 23, 11–17. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Crespo-Alonso, M.; Zoroddu, M.A.; Peana, M. Kill or Cure: Misuse of Chelation Therapy for Human Diseases. Coord. Chem. Rev. 2015, 284, 278–285. [Google Scholar] [CrossRef]
- Lamas, G.A.; Issa, O.M. Edetate Disodium-Based Treatment for Secondary Prevention in Post-Myocardial Infarction Patients. Curr. Cardiol. Rep. 2016, 18, 20. [Google Scholar] [CrossRef]
- Polyakov, N.; Leshina, T.; Fedenok, L.; Slepneva, I.; Kirilyuk, I.; Furso, J.; Olchawa, M.; Sarna, T.; Elas, M.; Bilkis, I.; et al. Redox-Active Quinone Chelators: Properties, Mechanisms of Action, Cell Delivery, and Cell Toxicity. Antioxid. Redox Signal. 2018, 28, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Świderski, G.; Krętowski, R.; Lewandowski, W. Newly Synthesized Doxorubicin Complexes with Selected Metals-Synthesis, Structure and Anti-Breast Cancer Activity. Molecules 2017, 22, 1106. [Google Scholar] [CrossRef] [PubMed]
- Hiller, M.C.; Anderson, W.F. Development of Iron Chelators for Clinical Use; Anderson, W.F., Hiller, M.C., Eds.; Bethesda, Md.: U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Institute of Arthritis, Metabolism, and Digestive Diseases: Maryland, WA, USA, 1975; pp. 1–277. [Google Scholar]
- Aisen, P. Some Physicochemical Aspects of Iron Metabolism. Ciba Found. Symp. 1976, 51, 1–17. [Google Scholar]
- Kontoghiorghes, G. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia; University of Essex: Colchester, UK, 1982; Available online: https://www.pri.ac.cy/files/KGJ_thesis_1982.pdf (accessed on 28 July 2020).
- Kontoghiorghes, G.; Pattichis, K.; Neocleous, K.; Kolnagou, A. The Design and Development of Deferiprone (L1) and Other Iron Chelators for Clinical Use: Targeting Methods and Application Prospects. Curr. Med. Chem. 2004, 11, 2161–2183. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.W.; Workman, E.F.; Schlabach, M.R. Does Transferrin Exhibit Ferroxidase Activity? Biochem. Biophys. Res. Commun. 1973, 50, 84–90. [Google Scholar] [CrossRef]
- Huebers, H.A.; Josephson, B.; Huebers, E.; Csiba, E.; Finch, C.A. Occupancy of the Iron Binding Sites of Human Transferrin. Proc. Natl. Acad. Sci. USA 1984, 81, 4326–4330. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J. The Study of Iron Mobilisation from Transferrin Using α-ketohydroxy Heteroaromatic Chelators. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1986, 869, 141–146. [Google Scholar] [CrossRef]
- Baldwin, D.A.; Jenny, E.R.; Aisen, P. The Effect of Human Serum Transferrin and Milk Lactoferrin on Hydroxyl Radical Formation from Superoxide and Hydrogen Peroxide. J. Biol. Chem. 1984, 259, 13391–13394. [Google Scholar]
- Huebers, H.A.; Huebers, E.; Csiba, E.; Finch, C.A. Iron Uptake from Rat Plasma Transferrin by Rat Reticulocytes. J. Clin. Invest. 1978, 62, 944–951. [Google Scholar] [CrossRef]
- Matias, C.; Belnap, D.W.; Smith, M.T.; Stewart, M.G.; Torres, I.F.; Gross, A.J.; Watt, R.K. Citrate and Albumin Facilitate Transferrin Iron Loading in the Presence of Phosphate. J. Inorg. Biochem. 2017, 168, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Waltersdorph, A.M. Prooxidant Activity of Transferrin and Lactoferrin. J. Exp. Med. 1990, 172, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Iron Mobilisation from Lactoferrin by Chelators at Physiological pH. BBA Gen. Subj. 1986, 882, 267–270. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Paterson, S.K.; Segal, A.W.; Halliwell, B. Inhibition of Lipid Peroxidation by the Iron-Binding Protein Lactoferrin. Biochem. J. 1981, 199, 259–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofi-Baffoni, S.; Nasta, V.; Banci, L. Protein Networks in the Maturation of Human Iron-Sulfur Proteins. Metallomics 2018, 10, 49–72. [Google Scholar] [CrossRef]
- Sargent, P.; Farnaud, S.; Evans, R. Structure/Function Overview of Proteins Involved in Iron Storage and Transport. Curr. Med. Chem. 2005, 12, 2683–2693. [Google Scholar] [CrossRef]
- Mehlenbacher, M.; Poli, M.; Arosio, P.; Santambrogio, P.; Levi, S.; Chasteen, N.D.; Bou-Abdallah, F. Iron Oxidation and Core Formation in Recombinant Heteropolymeric Human Ferritins. Biochemistry 2017, 56, 3900–3912. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: The Protein Nanocage and Iron Biomineral in Health and in Disease. Inorg. Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef]
- Beinert, H.; Holm, R.H.; Münck, E. Iron-Sulfur Clusters: Nature’s Modular, Multipurpose Structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef]
- Jacobs, A. An Intracellular Transit Iron Pool. Ciba Found. Symp. 1976, 51, 91–106. [Google Scholar]
- Hershko, C.; Graham, G.; Bates, G.W.; Rachmilewitz, E.A. Non-Specific Serum Iron in Thalassaemia: An Abnormal Serum Iron Fraction of Potential Toxicity. Br. J. Haematol. 1978, 40, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Potential Clinical Applications of Chelating Drugs in Diseases Targeting Transferrin-Bound Iron and Other Metals. Expert Opin. Investig. Drugs 2013, 22, 591–618. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G. Iron Mobilization from Transferrin and Non-Transferrin-Bound-Iron by Deferiprone. Implications in the Treatment of Thalassemia, Anemia of Chronic Disease, Cancer and Other Conditions. Hemoglobin 2006, 30, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Djaldetti, M.; Fishman, P.; Notti, I.; Bessler, H. The Effect of Tetracycline Administration on Iron Absorption in Mice. Biomedicine 1981, 35, 150–152. [Google Scholar]
- Konstantinou, E.; Pashalidis, I.; Kolnagou, A.; Kontoghiorghes, G.J. Interactions of Hydroxycarbamide (Hydroxyurea) with Iron and Copper: Implications on Toxicity and Therapeutic Strategies. Hemoglobin 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Sheppard, L.N.; Kontoghiorghes, G.J. Competition between Deferiprone, Desferrioxamine and Other Chelators for Iron and the Effect of Other Metals. Drug Res. (Stuttg). 1993, 43, 659–663. [Google Scholar]
- Lane, D.J.R.; Richardson, D.R. The Active Role of Vitamin C in Mammalian Iron Metabolism: Much More Than Just Enhanced Iron Absorption! Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Transferrin Cycle and Clinical Roles of Citrate and Ascorbate in Improved Iron Metabolism. ACS Chem. Biol. 2019, 14, 893–900. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Graham Goddard, J.; Bardett, A.N.; Sheppard, L. Pharmacokinetic Studies in Humans with the Oral Iron Chelator 1, 2-Dimethyl-3-Hydroxypyrid-4-One. Clin. Pharmacol. Ther. 1990, 48, 255–261. [Google Scholar] [CrossRef]
- Al-Refaie, F.N.; Wickens, D.G.; Wonke, B.; Kontoghiorghes, G.J.; Hoffbrand, A.V. Serum Non-Transferrin-Bound Iron in Beta-Thalassaemia Major Patients Treated with Desferrioxamine and L1. Br. J. Haematol. 1992, 82, 431–436. [Google Scholar] [CrossRef]
- Iancu, T.C.; Deugnier, Y.; Halliday, J.W.; Powell, L.W.; Brissot, P. Ultrastructural Sequences during Liver Iron Overload in Genetic Hemochromatosis. J. Hepatol. 1997, 27, 628–638. [Google Scholar] [CrossRef]
- Kyriacou, K.; Michaelides, Y.; Senkus, R.; Simamonian, K.; Pavlides, N.; Antoniades, L.; Zambartas, C. Ultrastructural Pathology of the Heart in Patients with β-Thalassaemia Major. Ultrastruct. Pathol. 2000, 24, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Iancu, T.C. Ferritin and Hemosiderin in Pathological Tissues. Electron. Microsc. Rev. 1992, 5, 209–229. [Google Scholar] [CrossRef]
- Papakonstantinou, O.; Alexopoulou, E.; Economopoulos, N.; Benekos, O.; Kattamis, A.; Kostaridou, S.; Ladis, V.; Efstathopoulos, E.; Gouliamos, A.; Kelekis, N.L. Assessment of Iron Distribution between Liver, Spleen, Pancreas, Bone Marrow, and Myocardium By Means of R2 Relaxometry with Mri in Patients with β-Thalassemia Major. J. Magn. Reson. Imaging 2009, 29, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kolnagou, A.; Natsiopoulos, K.; Kleanthous, M.; Ioannou, A.; Kontoghiorghes, G.J. Liver iron and Serum Ferritin Levels Are Misleading for Estimating Cardiac, Pancreatic, Splenic and Total Body Iron Load in Thalassemia Patients: Factors Influencing the Heterogenic Distribution of Excess Storage Iron in Organs as Identified by MRI T2. Toxicol. Mech. Methods 2013, 23, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kolnagou, A.; Michaelides, Y.; Kontos, C.; Kyriacou, K.; Kontoghiorghes, G.J. Myocyte Damage and Loss of Myofibers Is the Potential Mechanism of Iron Overload Toxicity in Congestive Cardiac Failure in Thalassemia. Complete Reversal of the Cardiomyopathy and Normalization of Iron Load by Deferiprone. Hemoglobin 2008, 32, 17–28. [Google Scholar] [CrossRef]
- Pennell, D.J. T2* Magnetic Resonance and Myocardial Iron in Thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 373–378. [Google Scholar] [CrossRef]
- Boddaert, N.; Sang, K.H.L.Q.; Rötig, A.; Leroy-Willig, A.; Gallet, S.; Brunelle, F.; Sidi, D.; Thalabard, J.C.; Munnich, A.; Cabantchik, Z.I. Selective Iron Chelation in Friedreich Ataxia: Biologic and Clinical Implications. Blood 2007, 110, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain Iron Chelation by Deferiprone in a Phase 2 Randomised Double-Blinded Placebo Controlled Clinical Trial in Parkinson’s Disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef]
- Zorzi, G.; Zibordi, F.; Chiapparini, L.; Bertini, E.; Russo, L.; Piga, A.; Longo, F.; Garavaglia, B.; Aquino, D.; Savoiardo, M.; et al. Iron-Related MRI Images in Patients with Pantothenate Kinase-Associated Neurodegeneration (PKAN) Treated with Deferiprone: Results of a Phase II Pilot Trial. Mov. Disord. 2011, 26, 1756–1759. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System—Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Bozonet, S.M.; Carr, A.C. The Role of physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elste, V.; Troesch, B.; Eggersdorfer, M.; Weber, P. Emerging Evidence on Neutrophil Motility Supporting Its Usefulness to Define Vitamin C Intake Requirements. Nutrients 2017, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. Int. Rev. J. 2014, 5, 16–18. [Google Scholar] [CrossRef]
- Traber, M.G.; Buettner, G.R.; Bruno, R.S. The Relationship between Vitamin C Status, the Gut-Liver Axis, and Metabolic Syndrome. Redox Biol. 2019, 21, 101091. [Google Scholar] [CrossRef]
- Knight, J.; Madduma-Liyanage, K.; Mobley, J.A.; Assimos, D.G.; Holmes, R.P. Ascorbic Acid Intake and Oxalate Synthesis. Urolithiasis 2016, 44, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; McCall, C. The Role of Vitamin C in the Treatment of Pain: New Insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Moustarah, F.; Mohiuddin, S.S. Dietary Iron; StatPearls: Treasure Island, FL, USA, 2019. [Google Scholar]
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological Roles of Iron in Cardiovascular Disease. Curr. Drug Targets 2018, 19, 1068–1076. [Google Scholar] [CrossRef]
- Man, C.D.; Maideen, S.F.K.; Rashid, A. Knowledge, Attitude and Practice Towards Dietary Iron Among Patients with Thalassemia and Their Caregivers in Peninsular Malaysia. Med. J. Malays. 2019, 74, 365–371. [Google Scholar]
- Wessells, K.R.; Young, R.R.; Ferguson, E.L.; Ouédraogo, C.T.; Faye, M.T.; Hess, S.Y. Assessment of Dietary Intake and Nutrient Gaps, and Development of Food-Based Recommendations, Among Pregnant and Lactating Women in Zinder, Niger: An Optifood Linear Programming Analysis. Nutrients 2019, 11, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Rawal, S. Dietary Iron Intake, Iron Status, and Gestational Diabetes. Am. J. Clin. Nutr. 2017, 106, 1672–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic Acid: Chemistry, Biology and the Treatment of Cancer. Biochim. Biophys. Acta-Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Vasta, J.D.; Raines, R.T. Human Collagen Prolyl 4-Hydroxylase Is Activated by Ligands for Its Iron Center. Biochemistry 2016, 55, 3224–3233. [Google Scholar] [CrossRef] [Green Version]
- Nasr, S.H.; Kashtanova, Y.; Levchuk, V.; Markowitz, G.S. Secondary Oxalosis Due to Excess Vitamin C Intake. Kidney Int. 2006, 70, 1672. [Google Scholar] [CrossRef] [Green Version]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of High-Dose Intravenous Vitamin C on Inflammation in Cancer Patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sesso, H.D.; Glynn, R.J.; Christen, W.G.; Bubes, V.; Manson, J.A.E.; Buring, J.E.; Gaziano, J.M. Vitamin E and C Supplementation and Risk of Cancer in Men: Posttrial Follow-Up in the Physicians’ Health Study II randomized Trial. Am. J. Clin. Nutr. 2014, 100, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Vance, T.M.; Su, J.; Fontham, E.T.H.; Koo, S.I.; Chun, O.K. Dietary Antioxidants and Prostate Cancer: A Review. Nutr. Cancer 2013, 65, 793–801. [Google Scholar] [CrossRef]
- Ohno, S.; Ohno, Y.; Suzuki, N.; Soma, G.I.; Inoue, M. High-Dose Vitamin C (Ascorbic Acid) Therapy in the Treatment of Patients with Advanced Cancer. Anticancer Res. 2009, 29, 809–815. [Google Scholar]
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Poulsen, H.E.; Lykkesfeldt, J.; Mikines, K.J. Elimination of Ascorbic Acid after High-Dose Infusion in Prostate Cancer Patients: A Pharmacokinetic Evaluation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C Pharmacokinetics in Healthy Volunteers: Evidence for a Recommended Dietary Allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myriam, M.; Sabatier, M.; Steiling, H.; Williamson, G. Skin Bioavailability of Dietary Vitamin E, Carotenoids, Polyphenols, Vitamin C, Zinc and Selenium. Br. J. Nutr. 2006, 96, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, C.L.; Franklin, R.B. Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine. HSOA J. Hum. Endocrinol. 2016, 1, 5. [Google Scholar] [CrossRef]
- Marik, P.E. Hydrocortisone, Ascorbic Acid and Thiamine (HAT Therapy) for the Treatment of Sepsis. Focus on Ascorbic Acid. Nutrients 2018, 10, 1762. [Google Scholar] [CrossRef] [Green Version]
- Hager, D.N.; Hinson, J.S.; Rothman, R.E. Vitamin C for Sepsis and Acute Respiratory Failure. JAMA J. Am. Med. Assoc. 2020, 323, 791–792. [Google Scholar] [CrossRef]
- Lynch, S.R.; Cook, J.D. Interaction of Vitamin C and Iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.; Geevarghese, P.; Khaire, P.; Joshi, T.; Suryawanshi, A.; Mundada, S.; Pawar, S.; Farookh, A. Comparison of Therapeutic Efficacy of Ferrous Ascorbate and Iron Polymaltose Complex in Iron Deficiency Anemia in Children: A Randomized Controlled Trial. Indian J. Pediatr. 2019, 86, 1112–1117. [Google Scholar] [CrossRef]
- Chandra, J. Treating Iron Deficiency Anemia. Indian J. Pediatr. 2019, 86, 1085–1086. [Google Scholar] [CrossRef] [Green Version]
- Pachuta Węgier, L.; Kubiak, M.; Liebert, A.; Clavel, T.; Montagne, A.; Stennevin, A.; Roye, S.; Boudribila, A. Ferrous Sulfate Oral Solution in Young Children with Iron Deficiency Anemia. Pediatr. Int. 2020. [Google Scholar] [CrossRef]
- Pippard, M.J. Desferrioxamine-Induced Iron Excretion in Humans. Baillieres. Clin. Haematol. 1989, 2, 323–343. [Google Scholar] [CrossRef]
- Hussain, M.A.M.; Green, N.; Flynn, D.M.; Hoffbrand, A.V. Effect of Dose, Time, and Ascorbate on Iron Excretion after Subcutaneous Desferrioxamine. Lancet 1977, 1, 977–979. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Aldouri, M.A.; Hoffbrand, A.V.; Barr, J.; Wonke, B.; Kourouclaris, T.; Sheppard, L. Effective Chelation of Iron in β Thalassaemia with the Oral Chelator 1, 2-Dimethyl-3-Hydroxypyrid-4-One. Br. Med. J. (Clin. Res. Ed.) 1987, 295, 1509–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elalfy, M.S.; Saber, M.M.; Adly, A.A.M.; Ismail, E.A.; Tarif, M.; Ibrahim, F.; Elalfy, O.M. Role of Vitamin C as an Adjuvant Therapy to Different Iron Chelators in Young β-Thalassemia Major Patients: Efficacy and Safety in Relation to Tissue Iron Overload. Eur. J. Haematol. 2016, 96, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Conte, D.; Brunelli, L.; Ferrario, L.; Mandelli, C.; Quatrini, M.; Velio, P.; Bianchi, P.A. Effect of Ascorbic Acid on Desferrioxamine-Induced Urinary Iron Excretion in Idiopathic Hemochromatosis. Acta Haematol. 1984, 72, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Chelators Affecting Iron Absorption in Mice. Drug Res. (Stuttg). 1990, 40, 1332–1335. [Google Scholar]
- Iyengar, V.; Pullakhandam, R.; Nair, K.M. Dietary Ligands as Determinants of Iron-Zinc Interactions at the Absorptive Enterocyte. J. Food Sci. 2010, 75, 260–264. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and Phytic Acid Contribute to the Low Iron Bioavailability from Common Beans in Young Women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef]
- Sotelo, A.; González-Osnaya, L.; Snchez-Chinchillas, A.; Trejo, A. Role of Oxate, Phytate, Tannins and Cooking on Iron Bioavailability from Foods Commonly Consumed in Mexico. Int. J. Food Sci. Nutr. 2010, 61, 29–39. [Google Scholar] [CrossRef]
- Jaramillo, Á.; Briones, L.; Andrews, M.; Arredondo, M.; Olivares, M.; Brito, A.; Pizarro, F. Effect of Phytic acid, Tannic Acid and Pectin on Fasting Iron Bioavailability Both in the Presence and Absence of Calcium. J. Trace Elem. Med. Biol. 2015, 30, 112–117. [Google Scholar] [CrossRef]
- Moridani, M.Y.; O’Brien, P.J. Iron Complexes of Deferiprone and Dietary Plant Catechols as Cytoprotective Superoxide Radical Scavengers. Biochem. Pharmacol. 2001, 62, 1579–1585. [Google Scholar] [CrossRef]
- Saleem, M.M.; Wilson, M.T. Kinetic Studies on the Reduction of Cytochrome c. Reaction with Dihydroxy Conjugated Compounds (Catechols and Quinols). Biochem. J. 1982, 201, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, N.; Hasinoff, B. Iron Supplements: A Common Cause of Drug Interactions. Br. J. Clin. Pharmacol. 1991, 31, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Stewart, B.W.; et al. Carcinogenicity of Consumption of Red and Processed Meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Gasche, C.; Ahmad, T.; Tulassay, Z.; Baumgart, D.C.; Bokemeyer, B.; Büning, C.; Howaldt, S.; Stallmach, A. Ferric Maltol Is Effective in Correcting Iron Deficiency Anemia in Patients with Inflammatory Bowel Disease: Results from a Phase-3 Clinical Trial Program. Inflamm. Bowel Dis. 2015, 21, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, S.B.; Rodgers, G.M. Parenteral Iron Therapy Options. Am. J. Hematol. 2004, 76, 74–78. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Iaina, A.; Peer, G.; Kaplan, E.; Levi, B.A.; Frank, N.; Steinbruch, S.; Blum, M. Intravenous Iron Supplementation for the Treatment of the Anemia of Moderate to Severe Chronic Renal Failure Patients Not Receiving Dialysis. Am. J. Kidney Dis. 1996, 27, 234–238. [Google Scholar] [CrossRef]
- Golriz, F.; Donnelly, L.F.; Devaraj, S.; Krishnamurthy, R. Modern American Scurvy—Experience with Vitamin C Deficiency at a Large Children’s Hospital. Pediatr. Radiol. 2017, 47, 214–220. [Google Scholar] [CrossRef]
- Ceglie, G.; Macchiarulo, G.; Marchili, M.R.; Marchesi, A.; Rotondi Aufiero, L.; Di Camillo, C.; Villani, A. Scurvy: STILL a Threat in the Well-Fed First World? Arch. Dis. Child. 2019, 104, 381–383. [Google Scholar] [CrossRef]
- Khalife, R.; Grieco, A.; Khamisa, K.; Tinmouh, A.; McCudden, C.; Saidenberg, E. Scurvy, an Old Story in a New Time: The Hematologist’s Experience. Blood Cells Mol. Dis. 2019, 76, 40–44. [Google Scholar] [CrossRef]
- Mantadakis, E.; Chatzimichael, E.; Zikidou, P. Iron Deficiency Anemia in Children Residing in High and Low-Income Countries: Risk Factors, Prevention, Diagnosis and Therapy. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020041. [Google Scholar]
- Valenzuela, C.; Olivares, M.; Brito, A.; Hamilton-West, C.; Pizarro, F. Is a 40% Absorption of Iron from a Ferrous ascorbate Reference Dose Appropriate to Assess Iron Absorption Independent of Iron Status? Biol. Trace Elem. Res. 2013, 155, 322–326. [Google Scholar] [CrossRef]
- Tarng, D.C.; Huang, T.P.; Wei, Y.H. Erythropoietin and Iron: The Role of Ascorbic Acid. Nephrol. Dial. Transplant. 2001, 16, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, T.; DeVita, M.V.; Michelis, M.F. Oral Vitamin C Supplementation Reduces Erythropoietin Requirement in Hemodialysis Patients with Functional Iron Deficiency. Int. Urol. Nephrol. 2016, 48, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Tarng, D.C.; Liu, T.Y.; Huang, T.P. Protective Effect of Vitamin C on 8-hydroxy-2′-Deoxyguanosine Level in Peripheral Blood Lymphocytes of Chronic Hemodialysis Patients. Kidney Int. 2004, 66, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutteridge, J.M.C. Plasma Ascorbate Levels and Inhibition of the Antioxidant Activity of Caeruloplasmin. Clin. Sci. 1991, 81, 413–417. [Google Scholar] [CrossRef]
- Sourabh, S.; Bhatia, P.; Jain, R. Favourable Improvement in Haematological Parameters in Response to Oral Iron and Vitamin C Combination in Children with Iron Refractory Iron Deficiency Anemia (IRIDA) Phenotype. Blood Cells Mol. Dis. 2019, 75, 26–29. [Google Scholar] [CrossRef]
- Scheers, N.; Sandberg, A.S. Iron Transport through Ferroportin Is Induced by Intracellular Ascorbate and Involves IRP2 and HIF2α. Nutrients 2014, 3, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Hawula, Z.J.; Wallace, D.F.; Subramaniam, V.N.; Rishi, G. Therapeutic Advances in Regulating the Hepcidin/Ferroportin Axis. Pharmaceuticals 2019, 12, 170. [Google Scholar] [CrossRef] [Green Version]
- Casu, C.; Chessa, R.; Liu, A.; Gupta, R.; Drakesmith, H.; Fleming, R.; Ginzburg, Y.Z.; MacDonald, B.; Rivella, S. Minihepcidins Improve Ineffective Erythropoiesis and Splenomegaly in a New Mouse Model of Adult Beta-Thalassemia Major. Haematologica 2019, 105, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Girelli, D.; Busti, F. Replacing the Suppressed Hormone: Toward a Better Treatment for Iron Overload in β-Thalassemia Major? Haematologica 2020, 105, 1752–1754. [Google Scholar] [CrossRef] [PubMed]
- Wapnick, A.A.; Lynch, S.R.; Krawitz, P.; Seftel, H.C.; Charlton, R.W.; Bothwell, T.H. Effects of Iron Overload on Ascorbic Acid Metabolism. Br. Med. J. 1968, 3, 704–707. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.D.; Barry, W.E.; Hershko, C.; Fillet, G.; Finch, C.A. Iron Kinetics with Emphasis on Iron Overload. Am. J. Pathol. 1973, 72, 337–344. [Google Scholar] [PubMed]
- Modell, C.B.; Beck, J. Long-Term Desferrioxamine Therapy in Thalassemia. Ann. N. Y. Acad. Sci. 1974, 232, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Angelucci, E.; Giardini, C.; Baronciani, D.; Galimberti, M.; Polchi, P.; Albertini, F.; Bartolucci, M.; Muretto, P. Fate of Iron Stores in Thalassaemia after Bone-Marrow Transplantation. Lancet 1993, 342, 1388–1391. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Compliance with Iron-Chelation Treatment after Bone Marrow Transplantation. Lancet 1994, 343, 604–605. [Google Scholar] [PubMed]
- Kontoghiorghes, G.J. Advances on Chelation and Chelator Metal Complexes in Medicine. Int. J. Mol. Sci. 2020, 21, 2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic Effects of the Lipophilic Iron Chelator Omadine. FEBS Lett. 1986, 204, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic and DNA-Inhibitory Effects of Iron Chelators on Human Leukaemic Cell Lines. Hematol. Oncol. 1986, 4, 195–204. [Google Scholar] [CrossRef]
- Hadi, S.M.; Ullah, M.F.; Shamim, U.; Bhatt, S.H.; Azmi, A.S. Catalytic Therapy of Cancer by Ascorbic Acid Involves Redox Cycling of Exogenous/Endogenous Copper Ions and Generation of Reactive Oxygen Species. Chemotherapy 2010, 56, 280–284. [Google Scholar] [CrossRef]
- Antholine, W.E.; Myers, C.R. Concentration of Fe(3+)-Triapine in Beas-2b Cells. Int. J. Mol. Sci. 2019, 20, 3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoghiorghes, G.J.; May, A. Uptake and Intracellular Distribution of Iron from Transferrin and Chelators in Erythroid Cells. Biol. Met. 1990, 3, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Tuntawiroon, M.; Sritongkul, N.; Brune, M.; Rossander-Hulten, L.; Pleehachinda, R.; Suwanik, R.; Hallberg, L. Dose-Dependent Inhibitory Effect of Phenolic Compounds in Foods on Nonheme-Iron Absorption in Men. Am. J. Clin. Nutr. 1991, 53, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Mostert, L.J.; Van Dorst, J.A.L.M.; Koster, J.F.; van Eijk, H.G.; Kontoghiorghes, G.J. Free Radical and Cytotoxic Effects of Chelators and Their Iron Complexes in the Hepatocyte. Free Radic. Res. 1987, 3, 379–388. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Andreou, N.; Constantinou, K.; Kontoghiorghes, G.J. World Health Dilemmas: Orphan and Rare Diseases, Orphan Drugs and Orphan Patients. World J. Methodol. 2014, 4, 163–188. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kleanthous, M.; Kontoghiorghe, C.N. The History of Deferiprone (L1) and the Paradigm of the Complete Treatment of Iron Overload in Thalassaemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020011. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines 2020, 7, 45. https://doi.org/10.3390/medicines7080045
Kontoghiorghes GJ, Kolnagou A, Kontoghiorghe CN, Mourouzidis L, Timoshnikov VA, Polyakov NE. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines. 2020; 7(8):45. https://doi.org/10.3390/medicines7080045
Chicago/Turabian StyleKontoghiorghes, George J., Annita Kolnagou, Christina N. Kontoghiorghe, Loukia Mourouzidis, Viktor A. Timoshnikov, and Nikolay E. Polyakov. 2020. "Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications" Medicines 7, no. 8: 45. https://doi.org/10.3390/medicines7080045
APA StyleKontoghiorghes, G. J., Kolnagou, A., Kontoghiorghe, C. N., Mourouzidis, L., Timoshnikov, V. A., & Polyakov, N. E. (2020). Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines, 7(8), 45. https://doi.org/10.3390/medicines7080045