Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease
Abstract
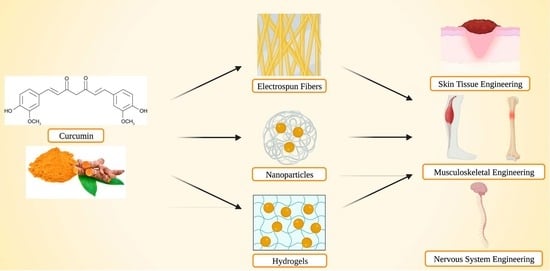
:1. Introduction
2. Curcumin
3. Curcumin Delivery from Electrospun Fibers
3.1. Skin Tissue Engineering
3.1.1. Full-Thickness Wound Healing Models
3.1.2. Diabetic Wounds
3.2. Bone Tissue Engineering
4. Curcumin Delivery from Nanoparticles
4.1. Skin Tissue Engineering
4.1.1. Full-Thickness Wound Healing Models
4.1.2. General Wound Healing Models
4.2. Musculoskeletal Engineering
Tendon Rupture and Repair Model
4.3. Nervous System Engineering
4.3.1. Huntington’s Disease
4.3.2. Alzheimer’s Disease
4.3.3. Stroke Model
4.3.4. Subarachnoid Hemorrhage Model
4.3.5. Traumatic Brain Injury Model
5. Curcumin Delivery from Hydrogels
5.1. Skin Tissue Engineering
5.1.1. Full-Thickness Wound Models
5.1.2. General Wound Healing Models
5.1.3. Diabetic Wounds
5.1.4. Burn Wounds
5.2. Bone Tissue Engineering
5.3. Central Nervous System Engineering
5.3.1. Spinal Cord Injury
5.3.2. Traumatic Brain Injury
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-0713-2. [Google Scholar]
- Aggarwal, B.B.; Takada, Y.; Oommen, O.V. From Chemoprevention to Chemotherapy: Common Targets and Common Goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef]
- Araújo, C.C.; Leon, L.L. Biological Activities of Curcuma Longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From Ancient Medicine to Current Clinical Trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Health Benefits, Extraction and Development of Functional Foods with Curcuminoids. J. Funct. Foods 2021, 79, 104392. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A Protective Effect of Curcumin on Cardiovascular Oxidative Stress Indicators in Systemic Inflammation Induced by Lipopolysaccharide in Rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by Curcumin: A Review on Brain Delivery Strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef]
- Barandeh, B.; Amini Mahabadi, J.; Azadbakht, M.; Gheibi Hayat, S.M.; Amini, A. The Protective Effects of Curcumin on Cytotoxic and Teratogenic Activity of Retinoic Acid in Mouse Embryonic Liver. J. Cell. Biochem. 2019, 120, 19371–19376. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yan, L.; Lou, Y.; Ying, L. The Protective Effect of Curcumin on Testicular Tissue in a Cryptorchid Rat Model. J. Pediatr. Urol. 2022, 18, 409.e1–409.e7. [Google Scholar] [CrossRef]
- Hong, J. Protective Effects of Curcumin-Regulated Intestinal Epithelial Autophagy on Inflammatory Bowel Disease in Mice. Gastroenterol. Res. Pract. 2022, 2022, e2163931. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cho, D.-C.; Han, I.; Kim, K.-T. Curcumin as a Promising Neuroprotective Agent for the Treatment of Spinal Cord Injury: A Review of the Literature. Neurospine 2022, 19, 249–261. [Google Scholar] [CrossRef]
- Tahereh, F.; Saeed, S. Antidotal Effects of Curcumin Against Agents-Induced Cardiovascular Toxicity. Cardiovasc. Hematol. Disord.-Drug Targets 2016, 16, 30–37. [Google Scholar]
- Hesari, A.; Azizian, M.; Sheikhi, A.; Nesaei, A.; Sanaei, S.; Mahinparvar, N.; Derakhshani, M.; Hedayt, P.; Ghasemi, F.; Mirzaei, H. Chemopreventive and Therapeutic Potential of Curcumin in Esophageal Cancer: Current and Future Status. Int. J. Cancer 2019, 144, 1215–1226. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Yuandani; Jantan, I.; Rohani, A.S.; Sumantri, I.B. Immunomodulatory Effects and Mechanisms of Curcuma Species and Their Bioactive Compounds: A Review. Front. Pharmacol. 2021, 12, 643119. [Google Scholar] [CrossRef]
- Culibrk, R.A.; Hahn, M.S. The Role of Chronic Inflammatory Bone and Joint Disorders in the Pathogenesis and Progression of Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 583884. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Extracellular Matrix, Supramolecular Organisation and Shape. J. Anat. 1995, 187, 259–269. [Google Scholar] [PubMed]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, S20–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, T.J.; Badylak, S.F. Biomaterials for Tissue Engineering Applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun Biomaterials in the Treatment and Prevention of Scars in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 481. [Google Scholar] [CrossRef]
- Lyu, S.; Huang, C.; Yang, H.; Zhang, X. Electrospun Fibers as a Scaffolding Platform for Bone Tissue Repair. J. Orthop. Res. 2013, 31, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Patel, M.; Patel, R. Electrospun Nanofiber Nerve Guidance Conduits for Peripheral Nerve Regeneration: A Review. Eur. Polym. J. 2022, 181, 111663. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of Injectable Hydrogel-Based Scaffolds for Cartilage Regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-Inspired Agarose Hydrogel Scaffolds for Skin Tissue Engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Oskouie, M.N.; Aghili Moghaddam, N.S.; Butler, A.E.; Zamani, P.; Sahebkar, A. Therapeutic Use of Curcumin-Encapsulated and Curcumin-Primed Exosomes. J. Cell. Physiol. 2019, 234, 8182–8191. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent Developments in Curcumin and Curcumin Based Polymeric Materials for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Mateti, T.; Ramakrishna, S.; Laha, A. A Review on Curcumin-Loaded Electrospun Nanofibers and Their Application in Modern Medicine. JOM 2022, 74, 3392–3407. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.; Jaiswal, P.; Mishra, A. Role of Curcumin and Its Nanoformulations in Neurotherapeutics: A Comprehensive Review. J. Biochem. Mol. Toxicol. 2020, 34, e22478. [Google Scholar] [CrossRef]
- Flores, G. Curcuma Longa L. Extract Improves the Cortical Neural Connectivity during the Aging Process. Neural Regen. Res. 2017, 12, 875–880. [Google Scholar] [CrossRef]
- PubChem Curcumin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/969516 (accessed on 29 August 2022).
- Rege, S.A.; Arya, M.; Momin, S.A. Structure Activity Relationship of Tautomers of Curcumin: A Review. Ukr. Food J. 2019, 8, 45–60. [Google Scholar] [CrossRef]
- Xiang, D.-B.; Zhang, K.-Q.; Zeng, Y.-L.; Yan, Q.-Z.; Shi, Z.; Tuo, Q.-H.; Lin, L.-M.; Xia, B.-H.; Wu, P.; Liao, D.-F. Curcumin. Medicine 2020, 99, e18467. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is a Preferred Antioxidant Mechanism of Curcumin. Available online: https://pubs.acs.org/doi/pdf/10.1021/ja991446m (accessed on 29 August 2022).
- Tønnesen, H.H.; Karlsen, J. Studies on Curcumin and Curcuminoids. VI. Kinetics of Curcumin Degradation in Aqueous Solution. Z. Fur Lebensm.-Unters. Und-Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative Absorption of Curcumin Formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [Green Version]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the Solubility and Pharmacological Efficacy of Curcumin by Heat Treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical Solids as a Platform to Improve Solubility and Bioavailability. CrystEngComm. 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of Unnatural Glucosides of Curcumin with Drastically Enhanced Water Solubility by Cell Suspension Cultures of Catharanthus Roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Tiwari, V.K.; Prakash, P. Making of Water Soluble Curcumin to Potentiate Conventional Antimicrobials by Inducing Apoptosis-like Phenomena among Drug-Resistant Bacteria. Sci. Rep. 2020, 10, 14204. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucisik, M.H.; Küpcü, S.; Schuster, B.; Sleytr, U.B. Characterization of CurcuEmulsomes: Nanoformulation for Enhanced Solubility and Delivery of Curcumin. J. Nanobiotechnol. 2013, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasra, M.; Khiri, H.; Ali, H.; Abdallah, O. Formulation, in-Vitro Characterization and Clinical Evaluation of Curcumin in-Situ Gel for Treatment of Periodontitis. Drug Deliv. 2017, 24, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A Critical Review of Analytical Methods for Determination of Curcuminoids in Turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef]
- Fu, S.-Z.; Meng, X.-H.; Fan, J.; Yang, L.-L.; Wen, Q.-L.; Ye, S.-J.; Lin, S.; Wang, B.-Q.; Chen, L.-L.; Wu, J.-B.; et al. Acceleration of Dermal Wound Healing by Using Electrospun Curcumin-Loaded Poly(ε-Caprolactone)-Poly(Ethylene Glycol)-Poly(ε-Caprolactone) Fibrous Mats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 533–542. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Vandermeulen, G.; Préat, V. Combined Effect of PLGA and Curcumin on Wound Healing Activity. J. Control. Release 2013, 171, 208–215. [Google Scholar] [CrossRef]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin Nanoparticles Incorporated in PVA/Collagen Composite Films Promote Wound Healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Requejo-Aguilar, R.; Alastrue-Agudo, A.; Cases-Villar, M.; Lopez-Mocholi, E.; England, R.; Vicent, M.J.; Moreno-Manzano, V. Combined Polymer-Curcumin Conjugate and Ependymal Progenitor/Stem Cell Treatment Enhances Spinal Cord Injury Functional Recovery. Biomaterials 2017, 113, 18–30. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Alqahtani, A.A.; Ullah, I.; Khan, N.R.; Basit, H.M.; Iftikhar, T.; Wahab, A.; Ali, M.; Badar, M. Microwave-Assisted Physically Cross-Linked Chitosan-Sodium Alginate Hydrogel Membrane Doped with Curcumin as a Novel Wound Healing Platform. AAPS PharmSciTech 2022, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and In Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (ε-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Calce, E.; Verdoliva, V.; Saviano, M.; Maglione, V.; Di Pardo, A.; De Luca, S. Curcumin-Loaded Nanoparticles Based on Amphiphilic Hyaluronan-Conjugate Explored as Targeting Delivery System for Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8846. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin Nanoparticles Attenuate Neurochemical and Neurobehavioral Deficits in Experimental Model of Huntington’s Disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, M.; Fang, J.; Yang, M.; Zhang, S.; Yin, Y.; Li, D.; Mao, L.; Fu, X.; Hou, Y.; et al. Enhanced Therapeutic Potential of Nano-Curcumin Against Subarachnoid Hemorrhage-Induced Blood–Brain Barrier Disruption Through Inhibition of Inflammatory Response and Oxidative Stress. Mol. Neurobiol. 2017, 54, 1–14. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancement of Curcumin Wound Healing Ability by Complexation with 2-Hydroxypropyl-γ-Cyclodextrin in Sacran Hydrogel Film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, N.; Lin, L. Curcumin Suppresses Apoptosis and Inflammation in Hypoxia/Reperfusion-Exposed Neurons via Wnt Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e920445-1–e920445-8. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Selvi, R.T.; Prakash, J.; Venkataprasanna, K.S.; Prema, D.; Pannerselvam, B.; Venkatasubbu, G.D. PVA/SA/TiO2-CUR Patch for Enhanced Wound Healing Application: In Vitro and In Vivo Analysis. Int. J. Biol. Macromol. 2019, 138, 704–717. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M.; Kashif, M.U.R. Curcumin-Laden Hyaluronic Acid-Co-Pullulan-Based Biomaterials as a Potential Platform to Synergistically Enhance the Diabetic Wound Repair. Int. J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef] [PubMed]
- El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Novel Curcumin-Loaded Gel-Core Hyaluosomes with Promising Burn-Wound Healing Potential: Development, In-Vitro Appraisal and In-Vivo Studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wu, Y.; Wu, Y.; Shi, K.; Han, R.; Li, Y.; Qian, Z.; Liao, J. Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-Co-Thermal Therapy and Bone Reconstruction. ACS Appl. Mater. Interfaces 2021, 13, 31542–31553. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An Injectable and Self-Healing Hydrogel with Controlled Release of Curcumin to Repair Spinal Cord Injury. Bioact. Mater. 2021, 6, 4816–4829. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Han, Y.; Han, Z.; Zhang, D.; Zhang, L.; Zhao, G.; Li, S.; Jin, G.; Yu, R.; Liu, H. In Situ Implantable, Post-Trauma Microenvironment-Responsive, ROS Depletion Hydrogels for the Treatment of Traumatic Brain Injury. Biomaterials 2021, 270, 120675. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Mouthuy, P.-A.; Ye, H. 5.04—Biomaterials: Electrospinning. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 23–36. ISBN 978-0-08-088504-9. [Google Scholar]
- Khil, M.-S.; Cha, D.-I.; Kim, H.-Y.; Kim, I.-S.; Bhattarai, N. Electrospun Nanofibrous Polyurethane Membrane as Wound Dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67B, 675–679. [Google Scholar] [CrossRef]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Rafienia, M.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Kolbuk, D.; Pahlevanneshan, Z.; Bonakdar, S.H. Development of Electrospun Poly (Vinyl Alcohol)-Based Bionanocomposite Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2018, 106, 1111–1120. [Google Scholar] [CrossRef]
- Wilhelm, K.-P.; Wilhelm, D.; Bielfeldt, S. Models of Wound Healing: An Emphasis on Clinical Studies. Ski. Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun Curcumin Loaded Poly(ε-Caprolactone)/Gum Tragacanth Nanofibers for Biomedical Application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin Loaded Poly(ε-Caprolactone) Nanofibers: Diabetic Wound Dressing with Antioxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Chapter 2—Biomaterials: Design, Development and Biomedical Applications. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, MS, USA, 2015; pp. 21–44. ISBN 978-0-323-32889-0. [Google Scholar]
- Khalid, S.A.; Abo Dena, A.S.; El-Sherbiny, I.M. Biopolymeric-Inorganic Composites for Drug Delivery Applications. In Polymeric and Natural Composites: Materials, Manufacturing and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Alkahtani, S., Eds.; Advances in Material Research and Technology; Springer International Publishing: Cham, Switzerland, 2022; pp. 271–298. ISBN 978-3-030-70266-3. [Google Scholar]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous Asymmetric Collagen/Curcumin Membrane Containing Aspirin-Loaded PLGA Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Liu, C.; Li, F.; Ouyang, C.; Gao, Q.; Zheng, K. Nanoparticles vs. Nanofibers: A Comparison of Two Drug Delivery Systems on Assessing Drug Release Performance In Vitro. Des. Monomers Polym. 2015, 18, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Jawahar, N.; Meyyanathan, S.N. Polymeric Nanoparticles for Drug Delivery and Targeting: A Comprehensive Review. Int. J. Health Allied Sci. 2012, 1, 217. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable Polymeric Nanoparticles as Drug Delivery Devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in Acute and Chronic Wound Healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional Regulation of NF-ΚB by Reactive Oxygen Species: A Role of Unfolded Protein Response. Free. Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of Curcumin Loaded Sodium Hyaluronate Immobilized Vesicles (Hyalurosomes) and Their Potential on Skin Inflammation and Wound Restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Frenkel, J.S. The Role of Hyaluronan in Wound Healing. Int. Wound J. 2012, 11, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic Roles of Hyaluronan in Dermal Wound Healing. Biomolecules 2021, 11, 1234. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Ahuja, S.; Chand, N.; Bajpai, M. Nano Cellulose Dispersed Chitosan Film with Ag NPs/Curcumin: An In Vivo Study on Albino Rats for Wound Dressing. Int. J. Biol. Macromol. 2017, 104, 1012–1019. [Google Scholar] [CrossRef]
- Liu, S.; Kang, Q.; Zhang, R.; Li, Y.; Bao, R.; Liu, S.; Kang, Q.; Zhang, R.; Li, Y.; Bao, R. Tendon Adhesion and Novel Solutions; IntechOpen: London, UK, 2022; ISBN 978-1-83768-186-0. [Google Scholar]
- Zhou, H.; Lu, H. Advances in the Development of Anti-Adhesive Biomaterials for Tendon Repair Treatment. Tissue Eng. Regen. Med. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ryoo, H.J.; Shim, H.-S. Prevention of Postoperative Adhesions after Flexor Tendon Repair with Acellular Dermal Matrix in Zones III, IV, and V of the Hand. Medicine 2022, 101, e28630. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Comes Franchini, M.; Xu, K.; Locatelli, E.; Martin, R.C.; Monaco, I.; Li, Y.; Cui, S. Controlled Release of Curcumin from Curcumin-Loaded Nanomicelles to Prevent Peritendinous Adhesion during Achilles Tendon Healing in Rats. Int. J. Nanomed. 2016, 11, 2873–2881. [Google Scholar] [CrossRef] [Green Version]
- Kazemi-Darabadi, S.; Nayebzadeh, R.; Shahbazfar, A.A.; Kazemi-Darabadi, F.; Fathi, E. Curcumin and Nanocurcumin Oral Supplementation Improve Muscle Healing in a Rat Model of Surgical Muscle Laceration. Bull. Emerg. Trauma 2019, 7, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A.; Akl, M.A.; Madkour, F.A. Effect of Chitosan and Curcumin Nanoparticles against Skeletal Muscle Fibrosis at Early Regenerative Stage of Glycerol-Injured Rat Muscles. BMC Musculoskelet. Disord. 2022, 23, 670. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Sarkar, S.; Jana, S.; Swarnakar, S.; Das, N. Neuro-Protective Role of Nanocapsulated Curcumin against Cerebral Ischemia-Reperfusion Induced Oxidative Injury. Brain Res. 2019, 1704, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Z.; Wu, S.-C.; Lin, C.-L.; Kwan, A.-L. Curcumin, Encapsulated in Nano-Sized PLGA, down-Regulates Nuclear Factor ΚB (P65) and Subarachnoid Hemorrhage Induced Early Brain Injury in a Rat Model. Brain Res. 2015, 1608, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Narouiepour, A.; Ebrahimzadeh-bideskan, A.; Rajabzadeh, G.; Gorji, A.; Negah, S.S. Neural Stem Cell Therapy in Conjunction with Curcumin Loaded in Niosomal Nanoparticles Enhanced Recovery from Traumatic Brain Injury. Sci. Rep. 2022, 12, 3572. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M.; Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications; IntechOpen: London, UK, 2016; ISBN 978-953-51-2510-5. [Google Scholar]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Lei, D.; Wang, Q.; Luo, X.; Chen, Y. Biocompatible Polyphosphorylcholine Hydrogels with Inherent Antibacterial and Nonfouling Behavior Effectively Promote Skin Wound Healing. ACS Appl. Bio Mater. 2020, 3, 5357–5366. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Singh, H.; Purohit, S.D.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Mishra, N.C. Curcumin in Decellularized Goat Small Intestine Submucosa for Wound Healing and Skin Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 210–219. [Google Scholar] [CrossRef]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound Healing Activity of Curcumin Conjugated to Hyaluronic Acid: In Vitro and In Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, L.H.; Nguyen, T.H.; Tran, H.L.B.; Doan, V.N.; Tran, N.Q. Injectable Nanocurcumin-Formulated Chitosan-g-Pluronic Hydrogel Exhibiting a Great Potential for Burn Treatment. J. Healthc. Eng. 2018, 2018, 5754890. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; Bonilla, P.; Giraldo, E.; Alastrue Agudo, A.; Edel, M.J.; Vicent, M.J.; Roca, F.G.; Ramos, C.M.; Doblado, L.R.; Pradas, M.M.; et al. A Hyaluronic Acid Demilune Scaffold and Polypyrrole-Coated Fibers Carrying Embedded Human Neural Precursor Cells and Curcumin for Surface Capping of Spinal Cord Injuries. Biomedicines 2021, 9, 1928. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Funnell, J.L.; Quinones, G.B.; Bentley, M.; Capadona, J.R.; Gilbert, R.J.; Palermo, E.F. Poly(pro-Curcumin) Materials Exhibit Dual Release Rates and Prolonged Antioxidant Activity as Thin Films and Self-Assembled Particles. Biomacromolecules 2023, 24, 294–307. [Google Scholar] [CrossRef]
- Forsyth, J.E.; Nurunnahar, S.; Islam, S.S.; Baker, M.; Yeasmin, D.; Islam, M.S.; Rahman, M.; Fendorf, S.; Ardoin, N.M.; Winch, P.J.; et al. Turmeric Means “Yellow” in Bengali: Lead Chromate Pigments Added to Turmeric Threaten Public Health across Bangladesh. Environ. Res. 2019, 179, 108722. [Google Scholar] [CrossRef]


| Method | Lowest Detection Limit [59] | Advantages | Limitations | Release Mediums | References |
|---|---|---|---|---|---|
| High-Performance Liquid Chromatography (HPLC) | 15 ng/mL |
|
| PBS | [60,61,62,63] |
| Ultra-High-Performance Liquid Chromatography (UHPLC) | 0.3 ng/mL |
|
|
| [64] |
| Ultraviolet-visible Spectrophotometry (UV-Vis) | 39 ng/mL |
|
| PBS; Water/Ethanol; Saline/Ethyl Alcohol | [65,66,67,68,69,70,71,72,73,74,75,76,77,78] |
| Ref. | Biomaterial Type(s) | Curcumin Incorporation Method | Electrospinning Parameters | Curcumin Release Kinetics | Model(s) | Significant Finding(s) |
|---|---|---|---|---|---|---|
| [60] | Poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCEC) Fibers | Blending |
| After 10 days:
|
| PCEC/Curcumin fibrous mats significantly increased wound closure compared to the untreated control |
| [65,87] | Poly(ε-caprolactone)/gum tragacanth (PCL/GT/Cur) Fibers | Blending |
| After 20 days,
|
| PCL/GT/Cur increased wound closure with well-formed granulation tissue |
| [88] | Poly(ε-caprolactone) (PCL) Fibers | Blending |
| After 3 days,
|
| 17%Cur-PCL fiber application accelerated wound closure and decreased inflammation compared to PCL nanofiber control |
| [91] * | Asymmetric Membrane: Collagen nanofiber and PLGA-aspirin nanoparticles (PACNFs); Curcumin and collagen nanofibers (CCNFs) | Blending |
| - |
| Overall composite material promoted new bone and soft tissue formation compared to commercial control |
| Ref. | Biomaterial Type(s) | Preparation Method | Drug Loading % | Encapsulation Efficiency % | Delivery Method | Curcumin Release Kinetics | Model(s) | Significant Finding(s) |
|---|---|---|---|---|---|---|---|---|
| [61] | Curcumin-loaded PLGA (PLGA-CC) | O/W Emulsion-Solvent Evaporation Technique | - | 89.2 ± 2.5% | Intradermal | After 8 days, 75.7 ± 3.4% |
| PLGA-CC promoted greater re-epithelialization and two-fold higher wound healing compared to control |
| [66] * | Electrospun PCL and Gelatin scaffold containing curcumin-loaded chitosan nanoparticles (PCL/Gela/NCs/Cur) | Solvent Evaporation | 4.2 ± 0.2% | 93 ± 5% | Topical | After 4 days, 100% (NCs/Cur) After 10 days, ~83% (PCL/Gela/NCs/Cur) |
| PCL/Gela/NCs/Cur demonstrated higher re-epithelialization, collagen synthesis, and wound healing |
| [99] | Curcumin-loaded hyalurosomes; curcumin-loaded liposomes | - | - | -Hyalurosomes: ~79% -Liposomes: ~66% (~54% after leakage) | Topical | - |
| Curcumin-loaded hyalurosomes reduced inflammation, edema, MPO activity, and promoted re-epithelialization |
| [102] * | Cellulose nanocrystals loaded chitosan films with curcumin/silver nanoparticles | - | - | - | Topical | - |
| Combination of curcumin with Ag nanoparticles greatly improved wound healing compared to curcumin alone |
| [62] * | Curcumin-loaded polyvinyl alcohol/collagen composite films (CPCF) | Solvent Evaporation | 9.61 ± 0.12% | 96.09 ± 1.21% | Topical | After 5 days, 90% (Cur NPs) 76% (CPCF) |
| CPCF treatment increased wound healing and epithelialization, as well as promotion of hair follicles |
| [107] | Curcumin-loaded nanomicelles (gold nanorods [GNRs]-1/curcumin in polymeric nanomicelles) (GNRs-1/curc@PMs) | Double Re-emulsification | - | 41% | Injection at tendon | - |
| GNRs-1/curc@PMs reduced peritendinous adhesions and demonstrated greater tendon strength with laser exposure |
| [108] | Curcumin-loaded nanomicelles (commercially available: SinaCurcumin) | - | - | - | Oral Gavage (100 mg/kg/day) | - |
| Curcumin-loaded nanomicelles had increased angiogenesis and muscle fiber regeneration following laceration injury |
| [109] | Curcumin-poly(ε-caprolactone) nanoparticles (Cn-NPs) | Single Emulsion-Solvent Evaporation | - | - | Intraperitoneal Injection | - |
| Cn-NPs reduced inflammation, decreased muscle fibrosis, and enhanced muscle regeneration following muscle injury |
| [67] | Curcumin-encapsulated hyaluronic acid-palmitate nanoparticles (Cur-HA-palmitate NPs) | Emulsification/Solvent Diffusion | - | - | After 72 h, ~70% |
| Cur-HA-palmitate NPs had greater cell penetration and reduced susceptibility to apoptosis | |
| [68] | Curcumin-encapsulated solid lipid nanoparticles (C-SLNs) | Solvent Evaporation | 93.25 ± 1.85% | - | Oral Gavage (40 mg/kg/day) | After 6 h, 53.77 ± 2.45% |
| C-SLNs increased mitochondrial activity, increased locomotor activity and reduced gait abnormalities |
| [69] | Curcumin-encapsulated PLGA nanoparticles (Cur-PLGA-NPs) | Emulsion-Solvent Evaporation | - | ~77 ± 5% | Intraperitoneal Injection | After ~36 h, ~60% After 7 days, ~74% |
| Cur-PLGA-NPs show greater reversal AD dysfunction via activation of Wnt/β-catenin pathway |
| [110] | Curcumin-incorporated PEGylated PLGA nanoparticles (NC) | Modified Emulsion-Diffusion-Evaporation | - | 58.9 ± 8.67% | Oral Gavage | After 24 h, ~30% After 48 h, ~44% |
| NC pre-treatment to CIR model had neuroprotective effects by reducing ROS-mediated damage and apoptosis |
| [70] | Poly(lactide-co-glycolide) (PLGA)-encapsulated curcumin nanoparticles (Cur-NPs) | Emulsification-Solvent-Diffusion | - | 81.7 ± 4.6% | Intraperitoneal Injection | After 12 h, 71.7 ± 4.1% After 36 h, 85.1 ± 3.5% |
| Cur-NPs attenuated blood-brain barrier dysfunction and glutamate concentrations, and reversed SAH-induced apoptosis |
| [111] | Curcumin-encapsulated PLGA nanoparticles | Two-step nanoprecipitation | - | - | Injection | - |
| Nanocurcumin decreased inflammation and reduced caspase-9 expression |
| [112] | Curcumin-loaded noisome nanoparticles | Thin-film hydration | - | - | Oral Gavage | - |
| CM-NPs combined with human neural stem/progenitor cells reduce brain edema and reduce inflammation |
| Ref. | Biomaterial Type(s) | Curcumin Incorporation Method | Delivery Method | Curcumin Release Kinetics | Model(s) | Significant Finding(s) |
|---|---|---|---|---|---|---|
| [71] * | Curcumin/2-hydroxypropyl-γ-cyclodextrin (HP-γ-CyD) complex in sacran-based hydrogel | Water Casting | Topical | After 24 h, 49.69 ± 3.74% After 120 h, 69.40 ± 5.16% | Hairless Mice
| High elastic modulus, Cur/HP-γ-CyD complex in Sac-HGF increased wound healing ability |
| [116] | Curcumin-loaded 2-(methacryloyloxy) ethyl 2-(trimethylammonio) ethyl phosphate copolymer (P(PC-co-GMA)) hydrogel (Cur-gel-G10M20) | Used as buffer solution in hydrogel preparation | Topical | After 24 h, 15% After 192 h, 48.5% | Sprague-Dawley Rats
| Cur-P(MPC-co-GMA) hydrogel increased wound healing rate and promoted reconstruction of hair follicles |
| [117] * | Curcumin-loaded micelles in a thermosensitive PEG-PCL-PEG hydrogel composite (Cur-M-H) | One-step solid dispersion into PEG-PCL copolymer | Topical | After 14 days, 40.1 ± 2.5% | Male Sprague-Dawley Rats
| In both wound models, Cur-M-H increased collagen formation, better granulation, and greater wound repair |
| [64] | Curcumin-loaded chitosan-sodium alginate hydrogel membrane | Dissolved into hydrogel solution | Topical | After 24 h, 41 ± 4.2% (Microwave-crosslinked) | Male Sprague-Dawley Rats
| Microwave-treated membrane promoted greater re-epithelialization with increased collagen deposition and greater epidermal definition |
| [73] * | Polyvinyl alcohol/sodium alginate/titanium dioxide-curcumin patch (PVA/SA/TiO2-Cur) | Synthesized to TiO2 to form a nanocomposite | Topical | After ~2.5 days, ~80% After 25 days, ~100% | Wistar Albino Rats
| PVA/SA/TiO2-Cur patch application increased wound healing and exhibited anti-bacterial properties against gram-positive and -negative bacteria |
| [118] | Curcumin-embedded decellularized goat small intestine submucosa (DG-SIS) hydrogel | Dissolved into scaffolds | - | DG-SIS/C3 After 5 h, 24% After 96 h, 73% | - | DG-SIS/C3 exhibited the greatest antibacterial properties, increased radical scavenging, and good biocompatibility |
| [74] | Curcumin-loaded hyaluronic-acid-Pullulan-g-F127 hydrogel (CUR-HA-Pu-g-F127) | Mixed into hyaluronic acid | Subcutaneous Injection | After 8 h, 50% After 24 h, ~84% |
| CUR-HA-Pu-g-F127 increased rate of wound healing and closure |
| [119] | Curcumin-conjugated hyaluronic acid hydrogel (HA-Cur) | Mixed into hyaluronic acid | Topical | - |
Full-Thickness Wound Model | HA-Cur revealed antibacterial properties, decreased oxidative damage, and increased wound healing |
| [75] | Curcumin-loaded gel-core hyalurosomes (Cur-GC-HS) | Gelled into Pluronic F-127 | Topical | After 2 h, ~50% After 6 h, ~81% | Female Sprague-Dawley Rats
| Cur-GC-HS increased wound healing with no scar formation, as well as higher skin deposition |
| [120] | Curcumin-loaded chitosan-g-pluronic copolymer nanocomposite hydrogel (nCur-CP) | Mixed into CP | Injection | - | Male Albino Mice
| nCur-CP enhanced wound closure, increased collagen density, thicker epidermis formation, and better granulation |
| [76] * | Curcumin-microsphere/IR820 coloaded hybrid methylcellulose hydrogel (Cur-MP/IR820) | Encapsulated into PLGA microspheres | Injection |
After 70 h, ~20%
After ~200 h, ~32% |
| Cur-MP/IR820 exhibited thermal-accelerated curcumin release and increased tumor cell apoptosis, osteogenic properties increasing bone reconstruction |
| [77] | Curcumin-loaded Fluorenylmethyloxycarbonyl protecting group (Fmoc)-grafted chitosan/Fmoc peptide hydrogel (FC/FI-Cur) | Dissolved into FC | Injection | After 48 h, ~68% After 168 h, ~82% | Female Sprague-Dawley Rats
| FC/FI-Cur accelerated DRG neurite outgrowth and SC migration in vitro. Modulation of inflammatory response, increased SC migration and remyelination in vivo |
| [121] * | Peptide hydrogel (HA-based with polypyrrole-coated fibers) (PM)-embedded human induced neural progenitor cells (iNPCs) with curcumin (PM-embedded iNPCs and CURC) | Mixed into PM | Local Placement over Spinal Cord | - | Female Sprague-Dawley Rats
| PPY-PM-iNPCs-CURC construct promotes neuron-like morphology in vitro and exhibits neuropreservation and decreases injured area in vivo |
| [63] | Curcumin-loaded polyacetal (PA) | Synthesized with PA | Intrathecal | pH 5.5 After ~180 h, ~100% pH 6.5 After ~180 h, ~50% | Sprague-Dawley Rats
| PA-curcumin increases neuroprotective effects and axonal growth, and promotes functional recovery with combined with epSPCs |
| [78] * | Curcumin-embedded matrix-metalloproteinase (MMP)-responsive triglycerol monostearate (TM) hydrogel | Embedded into poly(propylene sulfide)120 | Endocranium Placement | * Dependent on MMP activity and ROS in vitro After 14 days, ~80% (CSF) | Albino ICR Mice
| TM/PC reduced ROS and ROS-mediated effects, and brain edema, as well as exhibited anti-inflammatory effects; induced neuroregeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. https://doi.org/10.3390/bioengineering10020262
Hamilton AE, Gilbert RJ. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering. 2023; 10(2):262. https://doi.org/10.3390/bioengineering10020262
Chicago/Turabian StyleHamilton, Adelle E., and Ryan J. Gilbert. 2023. "Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease" Bioengineering 10, no. 2: 262. https://doi.org/10.3390/bioengineering10020262
APA StyleHamilton, A. E., & Gilbert, R. J. (2023). Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering, 10(2), 262. https://doi.org/10.3390/bioengineering10020262