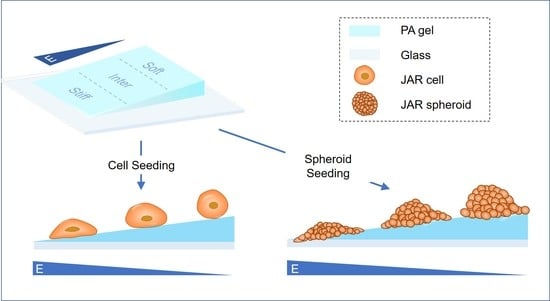
Stiff Extracellular Matrix Promotes Invasive Behaviors of Trophoblast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Cell Culture
2.2. JAR Spheroid Formation
2.3. Fabrication of Polyacrylamide Hydrogels with Stiffness Gradients
2.4. Cell Immunofluorescence
2.5. Image Analysis and Figure Preparation
2.6. Live Cell Imaging, Cell Tracking, and Migration Analyses
2.7. F-Actin Skeletonization
2.8. Focal Adhesion Area Measurement
2.9. Particle Image Velocimetry (PIV) Measurement
2.10. Durotaxis Assays
2.11. Spheroid Spreading Assay
2.12. Statistical Analysis
3. Results
3.1. Simulated ECM Stiffness Regulates Trophoblast Cell Morphology and Spreading Area
3.2. F-Actin Organization and Focal Adhesion Formation Are Affected by Simulated ECM Stiffness in JAR Cells
3.3. Stiff Simulated ECM Enhances JAR Cell Motility
3.4. JAR Cells Exhibit Durotaxis
3.5. Stiff Simulated ECM Enhances the Adhesion and Invasion of Multicellular JAR Spheroids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the Survival of Early Pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Foulk, R.A. Implantation of the Human Embryo; IntechOpen: London, UK, 2012. [Google Scholar]
- Fitzgerald, H.C.; Salamonsen, L.A.; Rombauts, L.J.; Vollenhoven, B.J.; Edgell, T.A. The proliferative phase underpins endometrial development: Altered cytokine profiles in uterine lavage fluid of women with idiopathic infertility. Cytokine 2016, 88, 12–19.f. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Garcia, J.; Kolp, L.; Cheadle, C.; Rodriguez, A.; Vlahos, N.F. The impact of luteal phase support on gene expression of extracellular matrix protein and adhesion molecules in the human endometrium during the window of implantation following controlled ovarian stimulation with a GnRH antagonist protocol. Fertil. Steril. 2010, 94, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qin, H.; Wang, H.; Liu, J.; Liu, S.; Yan, Q. N-glycosylation of uterine endometrium determines its receptivity. J. Cell. Physiol. 2020, 235, 1076–1089. [Google Scholar] [CrossRef]
- Ma, Z.; Sagrillo-Fagundes, L.; Mok, S.; Vaillancourt, C.; Moraes, C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci. Rep. 2020, 10, 5837. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef]
- Matsuzaki, S. Mechanobiology of the female reproductive system. Reprod. Med. Biol. 2021, 20, 371–401. [Google Scholar] [CrossRef]
- Kurek, A.; Kłosowicz, E.; Sofińska, K.; Jach, R.; Barbasz, J. Methods for Studying Endometrial Pathology and the Potential of Atomic Force Microscopy in the Research of Endometrium. Cells 2021, 10, 219. [Google Scholar] [CrossRef]
- Fang, S.; McLean, J.; Shi, L.; Vink, J.Y.; Hendon, C.P.; Myers, K.M. Anisotropic Mechanical Properties of the Human Uterus Measured by Spherical Indentation. Ann. Biomed. Eng. 2021, 49, 1923–1942. [Google Scholar] [CrossRef]
- Abbas, Y.; Carnicer-Lombarte, A.; Gardner, L.; Thomas, J.; Brosens, J.J.; Moffett, A.; Sharkey, A.M.; Franze, K.; Burton, G.J.; Oyen, M.L. Tissue stiffness at the human maternal-fetal interface. Hum. Reprod. 2019, 34, 1999–2008. [Google Scholar] [CrossRef] [Green Version]
- Di Pasquo, E.; Kiener, A.J.O.; DallAsta, A.; Commare, A.; Angeli, L.; Frusca, T.; Ghi, T. Evaluation of the uterine scar stiffness in women with previous Cesarean section by ultrasound elastography: A cohort study. Clin. Imaging 2020, 64, 53–56. [Google Scholar] [CrossRef]
- Kılıç, F.; Kayadibi, Y.; Yüksel, M.A.; Adaletli, İ.; Ustabaşıoğlu, F.E.; Öncül, M.; Madazlı, R.; Yılmaz, M.H.; Mihmanlı, İ.; Kantarcı, F. Shear wave elastography of placenta: In vivo quantitation of placental elasticity in preeclampsia. Diagn. Interv. Radiol. 2015, 21, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Asbach, P.; Streitberger, K.J.; Thomas, A.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. In vivo high-resolution magnetic resonance elastography of the uterine corpus and cervix. Eur. Radiol. 2014, 24, 3025–3033. [Google Scholar] [CrossRef]
- Thie, M.; Röspel, R.; Dettmann, W.; Benoit, M.; Ludwig, M.; Gaub, H.E.; Denker, H.W. Interactions between trophoblast and uterine epithelium: Monitoring of adhesive forces. Hum. Reprod. 1998, 13, 3211–3219. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.K.; Shawky, S.A.; Aryasomayajula, A.; Green, M.A.; Ewart, T.; Selvaganapathy, P.R.; Raha, S. Extracellular matrix surface regulates self-assembly of three-dimensional placental trophoblast spheroids. PLoS ONE 2018, 13, e0199632. [Google Scholar] [CrossRef]
- Kandow, C.E.; Georges, P.C.; Janmey, P.A.; Beningo, K.A. Polyacrylamide hydrogels for cell mechanics: Steps toward optimization and alternative uses. Methods Cell Biol. 2007, 83, 29–46. [Google Scholar]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Grümmer, R.; Hohn, H.P.; Mareel, M.M.; Denker, H.W. Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three-dimensional organ culture system. Placenta 1994, 15, 411–429. [Google Scholar] [CrossRef]
- Wang, H.; Pilla, F.; Anderson, S.; Martínez-Escribano, S.; Herrer, I.; Moreno-Moya, J.M.; Musti, S.; Bocca, S.; Oehninger, S.; Horcajadas, J.A. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol. Hum. Reprod. 2012, 18, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, C.; Yu, L.; Bocca, S.; Anderson, S.; Cunha-Filho, J.S.; Rhavi, B.S.; Oehninger, S. Role for the endometrial epithelial protein MFG-E8 and its receptor integrin αvβ3 in human implantation: Results of an in vitro trophoblast attachment study using established human cell lines. Fertil. Steril. 2014, 101, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Evron, A.; Goldman, S.; Shalev, E. Effect of primary human endometrial stromal cells on epithelial cell receptivity and protein expression is dependent on menstrual cycle stage. Hum. Reprod. 2011, 26, 176–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harduf, H.; Goldman, S.; Shalev, E. Human uterine epithelial RL95-2 and HEC-1A cell-line adhesiveness: The role of plexin B1. Fertil. Steril. 2007, 87, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Kodithuwakku, S.P.; Ng, P.Y.; Liu, Y.; Ng, E.H.; Yeung, W.S.; Ho, P.C.; Lee, K.F. Hormonal regulation of endometrial olfactomedin expression and its suppressive effect on spheroid attachment onto endometrial epithelial cells. Hum. Reprod. 2011, 26, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Heneweer, C.; Kruse, L.H.; Kindhäuser, F.; Schmidt, M.; Jakobs, K.H.; Denker, H.W.; Thie, M. Adhesiveness of human uterine epithelial RL95-2 cells to trophoblast: Rho protein regulation. Mol. Hum. Reprod. 2002, 8, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Heneweer, C.; Schmidt, M.; Denker, H.W.; Thie, M. Molecular mechanisms in uterine epithelium during trophoblast binding: The role of small GTPase RhoA in human uterine Ishikawa cells. J. Exp. Clin. Assist. Reprod. 2005, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Cai, P.; Layani, M.; Leow, W.R.; Amini, S.; Liu, Z.; Qi, D.; Hu, B.; Wu, Y.L.; Miserez, A.; Magdassi, S.; et al. Bio-Inspired Mechanotactic Hybrids for Orchestrating Traction-Mediated Epithelial Migration. Adv. Mater. 2016, 28, 3102–3110. [Google Scholar] [CrossRef]
- Guan, D.; Hang, Z.H.; Marcet, Z.; Liu, H.; Kravchenko, I.I.; Chan, C.T.; Chan, H.B.; Tong, P. Direct Measurement of Optical Force Induced by Near-Field Plasmonic Cavity Using Dynamic Mode AFM. Sci. Rep. 2015, 5, 16216. [Google Scholar] [CrossRef] [Green Version]
- Guan, D.; Shen, Y.; Zhang, R.; Huang, P.; Lai, P.-Y.; Tong, P. Unified description of compressive modulus revealing multiscale mechanics of living cells. Phys. Rev. Res. 2021, 3, 043166. [Google Scholar] [CrossRef]
- Hertz, H. On the contact of elastic solids. J. Reine Angew. Math. 1881, 92, 156–171. [Google Scholar]
- Gan, Z.; Ding, L.; Burckhardt, C.J.; Lowery, J.; Zaritsky, A.; Sitterley, K.; Mota, A.; Costigliola, N.; Starker, C.G.; Voytas, D.F.; et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 2016, 3, 252–263.e8. [Google Scholar] [CrossRef] [Green Version]
- Sigaut, L.; von Bilderling, C.; Bianchi, M.; Burdisso, J.E.; Gastaldi, L.; Pietrasanta, L.I. Live cell imaging reveals focal adhesions mechanoresponses in mammary epithelial cells under sustained equibiaxial stress. Sci. Rep. 2018, 8, 9788. [Google Scholar] [CrossRef] [Green Version]
- Shigehito, Y.; Mark, H.; Tetsuya, T. Human Embryology. In New Discoveries in Embryology; Bin, W., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 5. [Google Scholar]
- Kim, S.E.; Lee, J.E.; Han, Y.H.; Lee, S.I.; Kim, D.K.; Park, S.R.; Yu, S.L.; Kang, J. Decursinol from Angelica gigas Nakai enhances endometrial receptivity during implantation. BMC Complement. Med. Ther. 2020, 20, 36. [Google Scholar] [CrossRef] [Green Version]
- Tee, S.Y.; Fu, J.; Chen, C.S.; Janmey, P. Cell shape and substrate rigidity both regulate cell stiffness. Biophys. J. 2011, 100, L25–L27. [Google Scholar] [CrossRef] [Green Version]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Nicolas, A.; Besser, A.; Safran, S.A. Dynamics of cellular focal adhesions on deformable substrates: Consequences for cell force microscopy. Biophys. J. 2008, 95, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef]
- Wormer, D.B.; Davis, K.A.; Henderson, J.H.; Turner, C.E. The focal adhesion-localized CdGAP regulates matrix rigidity sensing and durotaxis. PLoS ONE 2014, 9, e91815. [Google Scholar] [CrossRef] [Green Version]
- Califano, J.P.; Reinhart-King, C.A. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell. Mol. Bioeng. 2010, 3, 68–75. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, A.J.; Hicks, S.R.; Svec, K.V.; Naughton, H.; Edmunds, Z.L.; Howe, A.K. The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Sci. Rep. 2018, 8, 7228. [Google Scholar] [CrossRef] [Green Version]
- Pelham, R.J., Jr.; Wang, Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Actomyosin contractility and collective migration: May the force be with you. Curr. Opin. Cell Biol. 2017, 48, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J. Biomech. Eng. 2017, 139, 0210051–02100519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffhauer, E.S.; Robinson, D.N. Mechanochemical Signaling Directs Cell-Shape Change. Biophys. J. 2017, 112, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Stroka, K.M.; Konstantopoulos, K. Physical biology in cancer. 4. Physical cues guide tumor cell adhesion and migration. Am. J. Physiol. Cell Physiol. 2014, 306, C98–C109. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, G.; Luo, X.; Qiu, J.; Tang, C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns 2012, 38, 414–420. [Google Scholar] [CrossRef]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, S.V.; Waterman, C.M. Guiding cell migration by tugging. Curr. Opin. Cell Biol. 2013, 25, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Aubry, D.; Gupta, M.; Ladoux, B.; Allena, R. Mechanical link between durotaxis, cell polarity and anisotropy during cell migration. Phys. Biol. 2015, 12, 026008. [Google Scholar] [CrossRef] [Green Version]
- Roca-Cusachs, P.; Sunyer, R.; Trepat, X. Mechanical guidance of cell migration: Lessons from chemotaxis. Curr. Opin. Cell Biol. 2013, 25, 543–549. [Google Scholar] [CrossRef]
- DuChez, B.J. Durotaxis by Human Cancer Cells. Biophys. J. 2019, 116, 670–683. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.B.; Brady, S.W.; Tripathi, A.; Hoffman-Kim, D. Schwann cell durotaxis can be guided by physiologically relevant stiffness gradients. Biomater. Res. 2018, 22, 14. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.S.; Schlenoff, J.B.; Keller, T.C., 3rd. Collective epithelial cell sheet adhesion and migration on polyelectrolyte multilayers with uniform and gradients of compliance. Exp. Cell Res. 2016, 346, 17–29. [Google Scholar]
- Shellard, A.; Mayor, R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature 2021, 600, 690–694. [Google Scholar] [CrossRef]
- Murray, M.J.; Lessey, B.A. Embryo implantation and tumor metastasis: Common pathways of invasion and angiogenesis. Semin. Reprod. Endocrinol. 1999, 17, 275–290. [Google Scholar] [CrossRef]
- Pilka, R.; Kudela, M.; Procházka, M. Matrix metalloproteinases, embryo implantation and tumor invasion. Ceska Gynekol. 2003, 68, 179–185. [Google Scholar]
- Soundararajan, R.; Rao, A.J. Trophoblast ‘pseudo-tumorigenesis’: Significance and contributory factors. Reprod. Biol. Endocrinol. 2004, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Lamptey, J.; Czika, A.; Aremu, J.O.; Pervaz, S.; Adu-Gyamfi, E.A.; Otoo, A.; Li, F.; Wang, Y.X.; Ding, Y.B. The role of fascin in carcinogenesis and embryo implantation. Exp. Cell Res. 2021, 409, 112885. [Google Scholar] [CrossRef]
- Grasset, E.M.; Bertero, T.; Bozec, A.; Friard, J.; Bourget, I.; Pisano, S.; Lecacheur, M.; Maiel, M.; Bailleux, C.; Emelyanov, A.; et al. Matrix Stiffening and EGFR Cooperate to Promote the Collective Invasion of Cancer Cells. Cancer Res. 2018, 78, 5229–5242. [Google Scholar] [CrossRef] [Green Version]
- Vinci, M.; Box, C.; Eccles, S.A. Three-dimensional (3D) tumor spheroid invasion assay. J. Vis. Exp. 2015, e52686. [Google Scholar]
- Timor-Tritsch, I.E.; Monteagudo, A.; Calì, G.; D’Antonio, F.; Kaelin Agten, A. Cesarean Scar Pregnancy: Diagnosis and Pathogenesis. Obstet. Gynecol. Clin. N. Am. 2019, 46, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 2020, 26, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Soffritti, I.; D’Accolti, M.; Gentili, V.; Di Luca, D.; Rizzo, R.; Caselli, E. HHV-6A Infection of Endometrial Epithelial Cells Affects miRNA Expression and Trophoblast Cell Attachment. Reprod. Sci. 2020, 27, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, U.K.; Chadchan, S.B.; Gupta, R.K.; Kumar, V.; Kumar Jha, R. miRNA-149 targets PARP-2 in endometrial epithelial and stromal cells to regulate the trophoblast attachment process. Mol. Hum. Reprod. 2021, 27, gaab039. [Google Scholar] [CrossRef]
- Ma, H.; Wang, J.; Zhao, X.; Wu, T.; Huang, Z.; Chen, D.; Liu, Y.; Ouyang, G. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020, 30, 793–806.e6. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhang, X.; Zhou, J.; Li, W.; Shu, X.; Wu, Y.; Long, M. Multiscale biomechanics and mechanotransduction from liver fibrosis to cancer. Adv. Drug Deliv. Rev. 2022, 188, 114448. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Z.; Cui, L.; Ban, Y.; Leung, P.C.K.; Li, Y.; Ma, J. Activin A increases human trophoblast invasion by upregulating integrin β1 through ALK4. FASEB J. 2021, 35, e21220. [Google Scholar] [CrossRef]
- Han, J.; Li, L.; Hu, J.; Yu, L.; Zheng, Y.; Guo, J.; Zheng, X.; Yi, P.; Zhou, Y. Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology 2010, 151, 1732–1742. [Google Scholar] [CrossRef]
- Shiokawa, S.; Iwashita, M.; Akimoto, Y.; Nagamatsu, S.; Sakai, K.; Hanashi, H.; Kabir-Salmani, M.; Nakamura, Y.; Uehata, M.; Yoshimura, Y. Small guanosine triphospatase RhoA and Rho-associated kinase as regulators of trophoblast migration. J. Clin. Endocrinol. Metab. 2002, 87, 5808–5816. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Li, H.; Tang, H.; Gu, X.; Wang, Y.; Guan, D.; Du, J.; Fan, Y. Stiff Extracellular Matrix Promotes Invasive Behaviors of Trophoblast Cells. Bioengineering 2023, 10, 384. https://doi.org/10.3390/bioengineering10030384
Cao J, Li H, Tang H, Gu X, Wang Y, Guan D, Du J, Fan Y. Stiff Extracellular Matrix Promotes Invasive Behaviors of Trophoblast Cells. Bioengineering. 2023; 10(3):384. https://doi.org/10.3390/bioengineering10030384
Chicago/Turabian StyleCao, Jialing, Hangyu Li, Hongyan Tang, Xuenan Gu, Yan Wang, Dongshi Guan, Jing Du, and Yubo Fan. 2023. "Stiff Extracellular Matrix Promotes Invasive Behaviors of Trophoblast Cells" Bioengineering 10, no. 3: 384. https://doi.org/10.3390/bioengineering10030384
APA StyleCao, J., Li, H., Tang, H., Gu, X., Wang, Y., Guan, D., Du, J., & Fan, Y. (2023). Stiff Extracellular Matrix Promotes Invasive Behaviors of Trophoblast Cells. Bioengineering, 10(3), 384. https://doi.org/10.3390/bioengineering10030384