Reliability of Retinal Layer Annotation with a Novel, High-Resolution Optical Coherence Tomography Device: A Comparative Study
Abstract
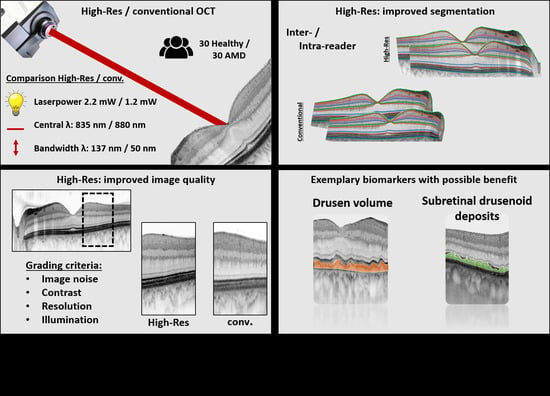
:1. Introduction
2. Methods
2.1. Subjects
2.2. Device Specifications
2.3. Imaging Protocol
2.4. Image Layer Annotation
2.5. Image Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.2. Retest Reliability of Retinal Layer Annotation
3.3. Image Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-Related Macular Degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Frcophth, U.C.; Spaide, R.F.; Nomenclature, I.; Tomography, C.; Oct, I.N. Proposed Lexicon for Anatomic Landmarks in Normal Posterior Segment Spectral-Domain Optical Coherence Tomography The IN OCT Consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef]
- Ooto, S.; Vongkulsiri, S.; Sato, T.; Suzuki, M.; Curcio, C.A.; Spaide, R.F. Outer Retinal Corrugations in Age-Related Macular Degeneration. JAMA Ophthalmol. 2014, 132, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: Literature Review and Model. RETINA 2011, 31, 1609–1619. [Google Scholar] [CrossRef]
- Mrejen, S.; Spaide, R.F. Optical Coherence Tomography: Imaging of the Choroid and Beyond. Surv. Ophthalmol. 2013, 58, 387–429. [Google Scholar] [CrossRef]
- Lavinsky, F.; Lavinsky, D. Novel Perspectives on Swept-Source Optical Coherence Tomography. Int. J. Retin. Vitr. 2016, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Caujolle, S.; Otto, T. Intermediate and deep capillary plexuses in machine learning segmentation of high-resolution optical coherence tomography imaging. RETINA 2021, 41, 1314–1317. [Google Scholar] [CrossRef]
- Podoleanu, A.; Bang, O.; Bojesen, S.; Bondu, M.; Bradu, A.; Caujolle, S.; Chin, C.; Denninger, M.; Feuchter, T.; Fleischhauer, F.; et al. Supercontinuum Applications in High Resolution Non Invasive Optical Imaging. In Proceedings of the Conference on Lasers and Electro-Optics; Optica Publishing Group: San Jose, CA, USA, 2018; p. AW3S.1. [Google Scholar]
- Wu, Z.; Pfau, M.; Blodi, B.A.; Holz, F.G.; Jaffe, G.J.; Liakopoulos, S.; Sadda, S.R.; Staurenghi, G.; Bjelopera, E.; Brown, T.; et al. OCT Signs of Early Atrophy in Age-Related Macular Degeneration: Interreader Agreement: Classification of Atrophy Meetings Report 6. Ophthalmol. Retin. 2022, 6, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Caujolle, S.; Cernat, R.; Silvestri, G.; Marques, M.J.; Bradu, A.; Feuchter, T.; Robinson, G.; Griffin, D.K.; Podoleanu, A. Speckle Variance OCT for Depth Resolved Assessment of the Viability of Bovine Embryos. Biomed. Opt. Express 2017, 8, 5139–5150. [Google Scholar] [CrossRef]
- Spaide, R.F.; Otto, T.; Caujolle, S.; Kübler, J.; Aumann, S.; Fischer, J.; Reisman, C.; Spahr, H.; Lessmann, A. Lateral Resolution of a Commercial Optical Coherence Tomography Instrument. Transl. Vis. Sci. Technol. 2022, 11, 28. [Google Scholar] [CrossRef]
- Wu, Z.; Luu, C.D.; Ayton, L.N.; Goh, J.K.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Optical Coherence Tomography-Defined Changes Preceding the Development of Drusen-Associated Atrophy in Age-Related Macular Degeneration. Ophthalmology 2014, 121, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Balaratnasingam, C.; Yannuzzi, L.A.; Curcio, C.A.; Morgan, W.H.; Querques, G.; Capuano, V.; Souied, E.; Jung, J.; Freund, K.B. Associations Between Retinal Pigment Epithelium and Drusen Volume Changes During the Lifecycle of Large Drusenoid Pigment Epithelial Detachments. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, M.; Malek, G.; Messinger, J.D.; Clark, M.E.; Wang, L.; Curcio, C.A. Sub-Retinal Drusenoid Deposits in Human Retina: Organization and Composition. Exp. Eye Res. 2008, 87, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; von der Emde, L.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Schmitz-Valckenberg, S.; Holz, F.G.; Fleckenstein, M.; Rubin, D.L. Progression of Photoreceptor Degeneration in Geographic Atrophy Secondary to Age-Related Macular Degeneration. JAMA Ophthalmol. 2020, 138, 1026–1034. [Google Scholar] [CrossRef]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.-Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. A Simplified Severity Scale for Age-Related Macular Degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef]
- Hess, K.; Gliem, M.; Charbel Issa, P.; Birtel, J.; Müller, P.L.; von der Emde, L.; Herrmann, P.; Holz, F.G.; Pfau, M. Mesopic and Scotopic Light Sensitivity and Its Microstructural Correlates in Pseudoxanthoma Elasticum. JAMA Ophthalmol. 2020, 138, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- von der Emde, L.; Pfau, M.; Dysli, C.; Thiele, S.; Moller, P.T.; Lindner, M.; Schmid, M.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Artificial Intelligence for Morphology-Based Function Prediction in Neovascular Age-Related Macular Degeneration. Sci. Rep. 2019, 9, 11132. [Google Scholar] [CrossRef]
- Pfau, M.; von der Emde, L.; Dysli, C.; Möller, P.T.; Thiele, S.; Lindner, M.; Schmid, M.; Rubin, D.L.; Fleckenstein, M.; Holz, F.G.; et al. Determinants of Cone- and Rod-Function in Geographic Atrophy: AI-Based Structure-Function Correlation. Am. J. Ophthalmol. 2020, 217, 162–173. [Google Scholar] [CrossRef]
- Sadigh, S.; Cideciyan, A.V.; Sumaroka, A.; Huang, W.C.; Luo, X.; Swider, M.; Steinberg, J.D.; Stambolian, D.; Jacobson, S.G. Abnormal Thickening as Well as Thinning of the Photoreceptor Layer in Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1603–1612. [Google Scholar] [CrossRef]
- Chiu, S.J.; Izatt, J.A.; O’Connell, R.V.; Winter, K.P.; Toth, C.A.; Farsiu, S. Validated Automatic Segmentation of AMD Pathology Including Drusen and Geographic Atrophy in SD-OCT Images. Investig. Ophthalmol. Vis. Sci. 2012, 53, 53–61. [Google Scholar] [CrossRef]
- Gonzalez Caldito, N.; Antony, B.; He, Y.; Lang, A.; Nguyen, J.; Rothman, A.; Ogbuokiri, E.; Avornu, A.; Balcer, L.; Frohman, E.; et al. Analysis of Agreement of Retinal-Layer Thickness Measures Derived from the Segmentation of Horizontal and Vertical Spectralis OCT Macular Scans. Curr. Eye Res. 2018, 43, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.N.; Smith, S.D.; Wright, M.M.; Grajewski, A.L.; Wang, Q.; Terry, J.M.; Lee, M.S. Reproducibility and Agreement in Evaluating Retinal Nerve Fibre Layer Thickness between Stratus and Spectralis OCT. Eye 2011, 25, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Leal, I.; Sousa, D.C.; Pinto, F.; Marques-Neves, C.; Abegão Pinto, L. Intra- and Inter-Rater Agreement of Anterior Lamina Cribrosa Depth Measurements Using Enhanced-Depth Imaging Optical Coherence Tomography. Ophthalmic Res. 2017, 57, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lujan, B.J.; Roorda, A.; Croskrey, J.A.; Dubis, A.M.; Cooper, R.F.; Bayabo, J.-K.; Duncan, J.L.; Antony, B.J.; Carroll, J. Directional Optical Coherence Tomography Provides Accurate Outer Nuclear Layer and Henle Fiber Layer Measurements. Retina 2015, 35, 1511–1520. [Google Scholar] [CrossRef]
- Grisso, P.; Pak, J.W.; de Silva, T.; Wiley, H.; Keenan, T.D.L.; Agron, E.; Chew, E.Y.; Cukras, C.A. Correlations Between AMD Severity and OCT Thickness. Investig. Ophthalmol. Vis. Sci. 2021, 62, 313. [Google Scholar]
- Farsiu, S.; O’Connell, R.V.; Chiu, S.J.; Winter, K.P.; Clark, L.A.; Tran-Viet, D.; Izatt, J.A.; Toth, C.A.; Group, A.S.S. Comprehensive Atlas of RPE-Drusen Complex Thickness Maps for Classification of Eyes with and without Intermediate AMD. Investig. Ophthalmol. Vis. Sci. 2012, 53, 844. [Google Scholar]
- Mayer, M.A.; Hornegger, J.; Mardin, C.Y.; Tornow, R.P. Retinal Nerve Fiber Layer Segmentation on FD-OCT Scans of Normal Subjects and Glaucoma Patients. Biomed. Opt. Express 2010, 1, 1358–1383. [Google Scholar] [CrossRef]
- Kafieh, R.; Rabbani, H.; Kermani, S. A Review of Algorithms for Segmentation of Optical Coherence Tomography from Retina. J. Med. Signals Sens. 2013, 3, 45–60. [Google Scholar]
- Varma, R.; Skaf, M.; Barron, E. Retinal Nerve Fiber Layer Thickness in Normal Human Eyes. Ophthalmology 1996, 103, 2114–2119. [Google Scholar] [CrossRef]
- Thiele, S.; Nadal, J.; Pfau, M.; Saßmannshausen, M.; von der Emde, L.; Fleckenstein, M.; Holz, F.G.; Schmid, M.; Schmitz-Valckenberg, S. Prognostic Value of Retinal Layers in Comparison with Other Risk Factors for Conversion of Intermediate Age-Related Macular Degeneration. Ophthalmol. Retin. 2020, 4, 31–40. [Google Scholar] [CrossRef]
- Borrelli, E.; Abdelfattah, N.S.; Uji, A.; Nittala, M.G.; Boyer, D.S.; Sadda, S.R. Postreceptor Neuronal Loss in Intermediate Age-Related Macular Degeneration. Am. J. Ophthalmol. 2017, 181, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Keltner, J.L.; Johnson, C.A.; Anderson, D.R.; Levine, R.A.; Fan, J.; Cello, K.E.; Quigley, H.A.; Budenz, D.L.; Parrish, R.K.; Kass, M.A. The Association between Glaucomatous Visual Fields and Optic Nerve Head Features in the Ocular Hypertension Treatment Study. Ophthalmology 2006, 113, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.Y.; Diniz-Filho, A.; Zangwill, L.M.; Gracitelli, C.P.B.; Marvasti, A.H.; Weinreb, R.N.; Baig, S.; Medeiros, F.A. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT421–OCT428. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.J.; Medeiros, F.A. Detecting Structural Progression in Glaucoma with Optical Coherence Tomography. Ophthalmology 2017, 124, S57–S65. [Google Scholar] [CrossRef]
- Leung, C.K.; Cheung, C.Y.; Weinreb, R.N.; Qiu, Q.; Liu, S.; Li, H.; Xu, G.; Fan, N.; Huang, L.; Pang, C.-P. Retinal Nerve Fiber Layer Imaging with Spectral-Domain Optical Coherence Tomography: A Variability and Diagnostic Performance Study. Ophthalmology 2009, 116, 1257–1263. [Google Scholar] [CrossRef]
- Curcio, C.A.; Allen, K.A. Topography of Ganglion Cells in Human Retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.V.; Liebmann, J.M.; Ritch, R. Glaucomatous Damage of the Macula. Prog. Retin. Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef]
- Liu, Z.; Kocaoglu, O.P.; Miller, D.T. 3D Imaging of Retinal Pigment Epithelial Cells in the Living Human Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT533–OCT543. [Google Scholar] [CrossRef]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef]
- Dansingani, K.K.; Balaratnasingam, C.; Naysan, J.; Freund, K.B. En Face Imaging of Pachychoroid Spectrum Disorders with Swept-Source Optical Coherence Tomography. Retina 2016, 36, 499–516. [Google Scholar] [CrossRef]
- Lauermann, J.L.; Treder, M.; Alnawaiseh, M.; Clemens, C.R.; Eter, N.; Alten, F. Automated OCT Angiography Image Quality Assessment Using a Deep Learning Algorithm. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Huang, D.; Wang, X.; Yu, M. Overview on Image Quality Assessment Methods. J. Electron. Inf. Technol. 2010, 32, 219–226. [Google Scholar] [CrossRef]



| AMD | Controls | |
|---|---|---|
| Participants | 30 | 30 |
| Age (years ± SD) | 75 ± 8 | 62 ± 17 |
| Sex female | 20 | 12 |
| Laterality right eye | 13 | 16 |
| Visual acuity (mean ± SD) | 0.2 ± 0.11 logMAR | 0.03 ± 0.05 logMAR |
| Mean Absolute Error of Retinal Layer Thickness Annotations [µm] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modality | Group | CHO | BM | RPE | IZ | EZ | ELM | OPL | INL | IPL | GCL | RNFL | ILM |
| High-Res OCT | AMD | 19.8 [4, 35] | 3.1 [2.3, 3.9] | 3.5 [2.8, 4.3] | 3.8 [3, 4.6] | 2.2 [1.9, 2.6] | 1.7 * [1.5, 2] | 2.9 [2.4, 3.6] | 2.5 [2.1, 2.9] | 2.9 * [2.5, 3.2] | 3.9 [3, 4.9] | 2.5 * [2.1, 2.9] | 1 [0.1, 1.9] |
| Control | 17.6 [11, 23] | 1.3 [0.4, 2.2] | 2.2 * [1.6, 2.9] | 2.5 [1.9, 3] | 1.3 * [1.1, 1.5] | 1 * [0.8,1.2] | 2.3 * [2.1, 2.6] | 2 * [1.5, 2.4] | 2.4 * [2.1, 2.7] | 3 * [2.5, 3.6] | 2.3 * [1.9, 2.6] | 0.1 [0, 0.5] | |
| Conventional_ OCT | AMD | 13.2 [6, 20] | 3.9 [2.2, 5.6] | 3.8 [3.2, 4.4] | 4.1 [3.3, 5] | 2.5 [2, 3.1] | 2.5 [1.8, 3.2] | 3.9 [2.4, 5.3] | 2.8 [2.3, 3.3] | 3.7 [3.1, 4.3] | 4.3 [3.7, 5] | 2.9 [2.5, 3.3] | 0.1 [0, 0.3] |
| Control | 21.1 [13, 28] | 1.3 [0.2, 2.4] | 3 [2.6, 3.4] | 2.6 [2.3, 2.8] | 1.7 [1.5, 1.9] | 1.6 [1.5, 1.7] | 3.1 [2.6, 3.6] | 2.8 [2.4, 3.2] | 3.2 [2.9, 3.5] | 4.7 [3.9, 4.5] | 2.8 [2.5, 3.1] | 0.4 [0, 1.3] | |
| Retinal Layers | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modality | Group | CHO | BM | RPE | IZ | EZ | ELM | OPL | INL | IPL | GCL | RNFL | ILM |
| High-Res OCT | AMD | 43.5 [29, 58] | 3.2 [2.2, 4.2] | 5.9 [4.4, 7.3] | 5.8 [5.1, 6.5] | 4.3 * [3.8, 4.7] | 3.1 [2.6, 3.6] | 5 * [4.4, 5.7] | 3.5 [3, 3.9] | 3.7 * [3.3, 4.1] | 5.8 * [4.9, 6.8] | 3 [2.6, 3.4] | 1.2 [0.4, 2.1] |
| Control | 54.1 [34, 73] | 0.7 [0.3, 1.2] | 3.3 [2.8, 3.8] | 3.9 [3.2, 4.5] | 4.4 * [4.1, 4.9] | 2.5 [2.2, 2.9] | 5.5 [4.7, 6.2] | 3.5 [2.9, 4.2] | 3.9 [3.5, 4.4] | 5.3 * [4.6, 6.1] | 2.7 * [2.4, 3] | 0.2 [0, 0.4] | |
| Conventional_ OCT | AMD | 38.8 [31, 47] | 3.9 [2.7, 5.1] | 6.4 [5.1, 7.7] | 6.5 [5.6, 7.3] | 6 [5.1, 6.9] | 3.2 [2.8, 3.7] | 6.3 [5.1, 7.5] | 3.9 [3.6, 4.3] | 4.4 [3.9, 4.8] | 9.1 [7.9, 10.2] | 3.3 [2.9, 3.6] | 0 [0, 0.1] |
| Control | 38.5 [31, 45] | 1 [0.2, 1.9] | 3.2 [2.7, 3.7] | 3.1 [2.7, 3.5] | 6.4 [5.8, 7] | 2.5 [2.2, 2.7] | 4.4 [3.7, 5.2] | 3.4 [3.1, 3.8] | 4.2 [3.7, 4.7] | 9.5 [8.2, 10.9] | 3.1 [2.7, 3.5] | 0.3 [0, 0.8] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von der Emde, L.; Saßmannshausen, M.; Morelle, O.; Rennen, G.; Holz, F.G.; Wintergerst, M.W.M.; Ach, T. Reliability of Retinal Layer Annotation with a Novel, High-Resolution Optical Coherence Tomography Device: A Comparative Study. Bioengineering 2023, 10, 438. https://doi.org/10.3390/bioengineering10040438
von der Emde L, Saßmannshausen M, Morelle O, Rennen G, Holz FG, Wintergerst MWM, Ach T. Reliability of Retinal Layer Annotation with a Novel, High-Resolution Optical Coherence Tomography Device: A Comparative Study. Bioengineering. 2023; 10(4):438. https://doi.org/10.3390/bioengineering10040438
Chicago/Turabian Stylevon der Emde, Leon, Marlene Saßmannshausen, Olivier Morelle, Geena Rennen, Frank G. Holz, Maximilian W. M. Wintergerst, and Thomas Ach. 2023. "Reliability of Retinal Layer Annotation with a Novel, High-Resolution Optical Coherence Tomography Device: A Comparative Study" Bioengineering 10, no. 4: 438. https://doi.org/10.3390/bioengineering10040438
APA Stylevon der Emde, L., Saßmannshausen, M., Morelle, O., Rennen, G., Holz, F. G., Wintergerst, M. W. M., & Ach, T. (2023). Reliability of Retinal Layer Annotation with a Novel, High-Resolution Optical Coherence Tomography Device: A Comparative Study. Bioengineering, 10(4), 438. https://doi.org/10.3390/bioengineering10040438