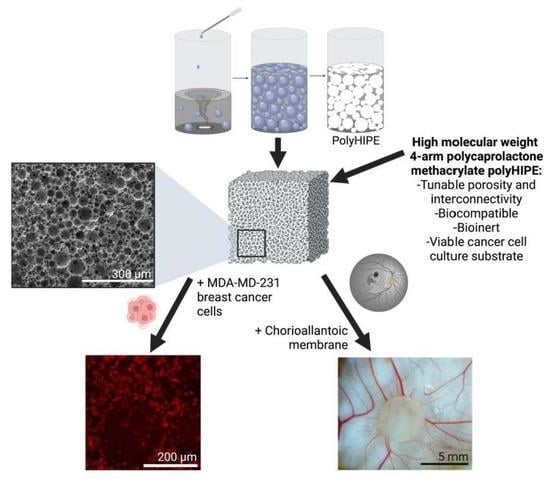
Development of PCL PolyHIPE Substrates for 3D Breast Cancer Cell Culture
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Preparation of PCL–M Emulsions
3.2. Polymerisation of PCL–M Emulsions
3.3. Assessment of PCL–M PolyHIPEs Porosity by SEM
3.4. Mechanical Characterisation
3.5. Surface Wettability of PCL–M polyHIPE
3.6. Assessment of Surface Functionalisation of PCL–M Scaffolds
3.7. General Cell Culture
3.8. Scaffold Fabrication for Cell Culture
3.9. MDA-MB-231 Cell Seeding on PCL–M polyHIPE Scaffolds
3.10. Cell Viability on PCL–M polyHIPE Scaffolds
3.11. CAM Assay
3.12. Statistical Analysis
4. Results
4.1. Manufacturing and Assessment of PCL–M polyHIPEs Porosity
4.2. Mechanical Characterisation of PCL–M polyHIPEs
4.3. Effect of Washing
4.4. Surface Wettability of PCL–M polyHIPE
4.5. Surface Functionalisation of PCL–M Scaffolds
4.6. Interaction of PCL–M polyHIPEs with a Vascular Network Using an Ex Ovo CAM Assay
4.7. Activity and Interaction of MDA-MB-231 Cells on PCL–M Scaffolds
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.M.; Hebert, K.L.; Gurrala, R.R.; Byrne, C.E.; Burow, M.; Martin, E.C.; Lau, F.H. Modeling Breast Cancer in Human Breast Tissue using a Microphysiological System. J. Vis. Exp. 2021, 2021, e62009. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Welsh, J.E. Animal Models for Studying Prevention and Treatment of Breast Cancer. In Animal Models for the Study of Human Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 997–1018. ISBN 9780124158948. [Google Scholar]
- Wolf, K.; Friedl, P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin. Exp. Metastasis 2009, 26, 289–298. [Google Scholar] [CrossRef]
- Bersini, S.; Jeon, J.S.; Moretti, M.; Kamm, R.D. In vitro models of the metastatic cascade: From local invasion to extravasation. Drug Discov. Today 2014, 19, 735–742. [Google Scholar] [CrossRef]
- Rijal, G.; Bathula, C.; Li, W. Application of Synthetic Polymeric Scaffolds in Breast Cancer 3D Tissue Cultures and Animal Tumor Models. Int. J. Biomater. 2017, 2017, 8074890. [Google Scholar] [CrossRef]
- Knight, E.; Murray, B.; Carnachan, R.; Przyborski, S. Alvetex®: Polystyrene Scaffold Technology for Routine Three Dimensional Cell Culture. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 695, pp. 323–340. [Google Scholar]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues–State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Przyborski, S.A.; Cameron, N.R. Emulsion-templated porous polymers as scaffolds for three dimensional cell culture: Effect of synthesis parameters on scaffold formation and homogeneity. J. Mater. Chem. 2007, 17, 4088–4094. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Novel cell culture device enabling three-dimensional cell growth and improved cell function. Biochem. Biophys. Res. Commun. 2007, 354, 1095–1100. [Google Scholar] [CrossRef]
- Hayman, M.W.; Smith, K.H.; Cameron, N.R.; Przyborski, S.A. Growth of human stem cell-derived neurons on solid three-dimensional polymers. J. Biochem. Biophys. Methods 2005, 62, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 479. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymer 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue Engineering of Bone: Material and Matrix Considerations. J. Bone Jt. Surg.-Am. Vol. 2008, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for in situ tissue regeneration: A review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Hung, K.C.; Chen, C.W. Biodegradable polymer scaffolds. J. Mater. Chem. B 2016, 4, 7493–7505. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Woodfield, T.B.F.; Dalton, P.D. Scaffold Design and Fabrication. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 311–346. ISBN 9780124201453. [Google Scholar]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.-S. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Sherborne, C.; Reilly, G.C.; Claeyssens, F. Emulsion templated scaffolds manufactured from photocurable polycaprolactone. Polymer 2019, 175, 243–254. [Google Scholar] [CrossRef]
- Cameron, N.R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439–1449. [Google Scholar] [CrossRef]
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Claeyssens, F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Moglia, R.S.; Holm, J.L.; Sears, N.A.; Wilson, C.J.; Harrison, D.M.; Cosgriff-Hernandez, E. Injectable PolyHIPEs as High Porosity Bone Grafts. Biomacromolecules 2011, 12, 3621. [Google Scholar] [CrossRef]
- Busby, W.; Cameron, N.R.; Jahoda, C.A.B. Emulsion-Derived Foams (PolyHIPEs) Containing Poly(E-caprolactone) as Matrixes for Tissue Engineering. Biomacromolecules 2001, 2, 154–164. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer–Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Dove, A.P. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 2016, 5, 9–21. [Google Scholar] [CrossRef]
- Diba, M.; Fathi, M.H.; Kharaziha, M. Novel forsterite/polycaprolactone nanocomposite scaffold for tissue engineering applications. Mater. Lett. 2011, 65, 1931–1934. [Google Scholar] [CrossRef]
- Dikici, B.A.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A novel bilayer polycaprolactone membrane for guided bone regeneration: Combining electrospinning and emulsion templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Ramos-rodriguez, D.H.; Macneil, S.; Claeyssens, F.; Asencio, I.O. Delivery of Bioactive Compounds to Improve Skin Cell Responses on Microfabricated Electrospun Microenvironments. Bioengineering 2021, 8, 105. [Google Scholar] [CrossRef]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ε-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; Cassan, D.; Menzel, H.; Hoheisel, A.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Wellmann, M.; et al. In vivo analysis of vascularization and biocompatibility of electrospun polycaprolactone fibre mats in the rat femur chamber. J. Tissue Eng. Regen. Med. 2019, 13, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Farr, N.T.H.; Klosterhalfen, B.; Noé, G.K. Characterization in respect to degradation of titanium-coated polypropylene surgical mesh explanted from humans. J. Biomed. Mater. Res. B. Appl. Biomater. 2023, 111, 1142–1152. [Google Scholar] [CrossRef]
- Barbetta, A.; Cameron, N.R. Morphology and surface area of emulsion-derived (PolyHIPE) solid foams prepared with oil-phase soluble porogenic solvents: Span 80 as surfactant. Macromolecules 2004, 37, 3188–3201. [Google Scholar] [CrossRef]
- Sigma Aldrich Authenticated MDA-MB-231 Cell Line. Available online: https://www.sigmaaldrich.com/GB/en/product/sigma/cb_92020424 (accessed on 12 August 2022).
- English, W.R.; Lunt, S.J.; Fisher, M.; Lefley, D.V.; Dhingra, M.; Lee, Y.-C.; Bingham, K.; Hurrell, J.E.; Lyons, S.K.; Kanthou, C.; et al. Differential Expression of VEGFA Isoforms Regulates Metastasis and Response to Anti-VEGFA Therapy in Sarcoma. Cancer Res. 2017, 77, 2633–2646. [Google Scholar] [CrossRef]
- Sandoval-Castellanos, A.M.; Claeyssens, F.; Haycock, J.W. Bioactive 3D Scaffolds for the Delivery of NGF and BDNF to Improve Nerve Regeneration. Front. Mater. 2021, 8, 466. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, H.; Chwee, T.L.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef]
- Dikici, S.; Aldemir Dikici, B.; Bhaloo, S.I.; Balcells, M.; Edelman, E.R.; MacNeil, S.; Reilly, G.C.; Sherborne, C.; Claeyssens, F. Assessment of the Angiogenic Potential of 2-Deoxy-D-Ribose Using a Novel in vitro 3D Dynamic Model in Comparison with Established in vitro Assays. Front. Bioeng. Biotechnol. 2020, 7, 20. [Google Scholar] [CrossRef]
- Naik, M.; Brahma, P.; Dixit, M. A cost-effective and efficient chick ex-ovo cam assay protocol to assess angiogenesis. Methods Protoc. 2018, 1, 19. [Google Scholar] [CrossRef]
- Mangir, N.; Dikici, S.; Claeyssens, F.; Macneil, S. Using ex Ovo Chick Chorioallantoic Membrane (CAM) Assay to Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Huš, S.; Krajnc, P. PolyHIPEs from Methyl methacrylate: Hierarchically structured microcellular polymers with exceptional mechanical properties. Polymer 2014, 55, 4420–4424. [Google Scholar] [CrossRef]
- Paterson, T.E.; Gigliobianco, G.; Sherborne, C.; Green, N.H.; Dugan, J.M.; Macneil, S.; Reilly, G.C.; Claeyssens, F. Porous microspheres support mesenchymal progenitor cell ingrowth and stimulate angiogenesis. APL Bioeng. 2018, 2, 026103. [Google Scholar] [CrossRef] [PubMed]
- Mravljak, R.; Bizjak, O.; Podlogar, M.; Podgornik, A. Effect of polyHIPE porosity on its hydrodynamic properties. Polym. Test. 2021, 93, 106590. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vagaská, B.; Bačáková, L.; Filová, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2010, 59, 309–322. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Mbundi, L.; González-Pérez, M.; González-Pérez, F.; Juanes-Gusano, D.; Rodríguez-Cabello, J.C. Trends in the Development of Tailored Elastin-Like Recombinamer–Based Porous Biomaterials for Soft and Hard Tissue Applications. Front. Mater. 2021, 7, 1–27. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Northey, J.J.; Barrett, A.S.; Acerbi, I.; Hayward, M.K.; Talamantes, S.; Dean, I.S.; Mouw, J.K.; Ponik, S.M.; Lakins, J.N.; Huang, P.J.; et al. Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. J. Clin. Investig. 2020, 130, 5721–5737. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In vitro tumor models: Advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. Part A 2006, 76, 264–271. [Google Scholar] [CrossRef]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Kwok, Y.K. Wettability on Different Surfaces. In 21st Century Surface Science—A Handbook; Pham, P., Goel, P., Kumar, S., Yadav, K., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-200-4. [Google Scholar]
- Farr, N.; Thanarak, J.; Schäfer, J.; Quade, A.; Claeyssens, F.; Green, N.; Rodenburg, C.; Farr, N.; Thanarak, J.; Claeyssens, F.; et al. Understanding Surface Modifications Induced via Argon Plasma Treatment through Secondary Electron Hyperspectral Imaging. Adv. Sci. 2021, 8, 2003762. [Google Scholar] [CrossRef]
- Puleo, D.A.; Kissling, R.A.; Sheu, M.S. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials 2002, 23, 2079–2087. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Cascardo, G.; Cahalan, P.; Cahalan, L.; Fini, M.; Giardino, R. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials 2003, 24, 4639–4654. [Google Scholar] [CrossRef]
- Friedrich, J.; Kühn, G.; Mix, R.; Fritz, A.; Schönhals, A. Polymer surface modification with monofunctional groups of variable types and densities. J. Adhes. Sci. Technol. 2012, 17, 1591–1617. [Google Scholar] [CrossRef]
- Cools, P.; Mota, C.; Lorenzo-Moldero, I.; Ghobeira, R.; De Geyter, N.; Moroni, L.; Morent, R. Acrylic Acid Plasma Coated 3D Scaffolds for Cartilage tissue engineering applications. Sci. Rep. 2018, 8, 3830. [Google Scholar] [CrossRef]
- Xing, H.; Li, R.; Wei, Y.; Ying, B.; Li, D.; Qin, Y. Improved Osteogenesis of Selective-Laser-Melted Titanium Alloy by Coating Strontium-Doped Phosphate With High-Efficiency Air-Plasma Treatment. Front. Bioeng. Biotechnol. 2020, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Carton, O.; Ben Salem, D.; Bhatt, S.; Pulpytel, J.; Arefi-Khonsari, F. Plasma Polymerization of Acrylic Acid by Atmospheric Pressure Nitrogen Plasma Jet for Biomedical Applications. Plasma Process. Polym. 2012, 9, 984–993. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, I.; Kanczler, J.M.; Hulsart-Billstrom, G.; Inglis, S.; Oreffo, R.O.C. *The Chorioallantoic Membrane Assay for Biomaterial Testing in Tissue Engineering: A Short-Term In Vivo Preclinical Model. Tissue Eng. Part C Methods 2017, 23, 938–952. [Google Scholar] [CrossRef]
- Baiguera, S.; Macchiarini, P.; Ribatti, D. Chorioallantoic membrane for in vivo investigation of tissue-engineered construct biocompatibility. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2012, 100, 1425–1434. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef]
- Bahmaee, H.; Owen, R.; Boyle, L.; Perrault, C.M.; Garcia-Granada, A.A.; Reilly, G.C.; Claeyssens, F. Design and Evaluation of an Osteogenesis-on-a-Chip Microfluidic Device Incorporating 3D Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 1042. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Cheng, M.H.; Engel, H.; Kao, S.W.; Larson, J.C.; Gupta, S.; Brey, E.M. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 2011, 32, 6045–6051. [Google Scholar] [CrossRef]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng.-Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef]







| C1s | C-C/C-H at% (285 eV) | C-O/C-N at% (286.2 eV) | (C,N)-C=O at% (288.2 eV) | HO-C=O at% (288.9 eV) |
|---|---|---|---|---|
| Untreated | 67.6 ± 0.1 | 19.1 ± 0.3 | 2.1 ± 0.2 | 11.3 ± 0.1 |
| Air plasma | 62.8 ± 3.0 | 22.4 ± 2.4 | 1.8 ± 0.4 | 13.1 ± 1.0 |
| AAc plasma | 56.3 ± 1.7 | 28.0 ±2.0 | 1.2 ± 0.1 | 14.6 ± 0.2 |
| Untreated and fibronectin | 61.4 ± 0.3 | 23.6 ± 0.3 | 4.1± 0.2 | 10.9 ± 0.9 |
| Air plasma and fibronectin | 52.7 ±2.6 | 29.3 ± 2.7 | 6.1 ± 0.1 | 11.8 ± 0.1 |
| AAc plasma and fibronectin | 55.6 ± 0.4 | 28.7 ± 0.3 | 6.2 ± 0.6 | 9.6 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, C.E.; Ramos-Rodriguez, D.H.; Farr, N.T.H.; English, W.R.; Green, N.H.; Claeyssens, F. Development of PCL PolyHIPE Substrates for 3D Breast Cancer Cell Culture. Bioengineering 2023, 10, 522. https://doi.org/10.3390/bioengineering10050522
Jackson CE, Ramos-Rodriguez DH, Farr NTH, English WR, Green NH, Claeyssens F. Development of PCL PolyHIPE Substrates for 3D Breast Cancer Cell Culture. Bioengineering. 2023; 10(5):522. https://doi.org/10.3390/bioengineering10050522
Chicago/Turabian StyleJackson, Caitlin E., David H. Ramos-Rodriguez, Nicholas T. H. Farr, William R. English, Nicola H. Green, and Frederik Claeyssens. 2023. "Development of PCL PolyHIPE Substrates for 3D Breast Cancer Cell Culture" Bioengineering 10, no. 5: 522. https://doi.org/10.3390/bioengineering10050522
APA StyleJackson, C. E., Ramos-Rodriguez, D. H., Farr, N. T. H., English, W. R., Green, N. H., & Claeyssens, F. (2023). Development of PCL PolyHIPE Substrates for 3D Breast Cancer Cell Culture. Bioengineering, 10(5), 522. https://doi.org/10.3390/bioengineering10050522