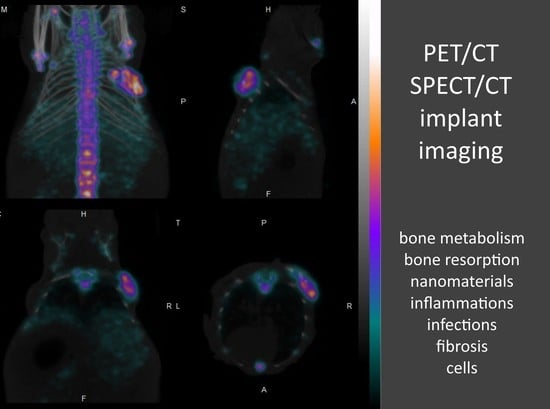
Implant Imaging: Perspectives of Nuclear Imaging in Implant, Biomaterial, and Stem Cell Research
Abstract
:1. Introduction
2. Bone Imaging: Quantitative Feedback on Bone Tissue Regeneration, Resorption, and Osseointegration
3. Infection, Inflammation, Biofilm, and Fibrosis Imaging
4. Cell and Stem Cell Tracking
5. Nanoparticle Imaging: Tracking Drug Delivery Systems and Drug Release
6. Pretargeting-Based Custom Biomaterial Imaging
7. Radiotheranostic Opportunities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Polyak, A.; Ross, T.L. Nanoparticles for SPECT and PET Imaging: Towards Personalized Medicine and Theranostics. Curr. Med. Chem. 2018, 25, 4328–4353. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Horvath, I.; Ferreira, S.; Lemos, J.; Costa, P.; Vieira, D.; Veres, D.S.; Szigeti, K.; Summavielle, T.; Máthé, D.; et al. Preclinical Imaging: An Essential Ally in Modern Biosciences. Mol. Diagn. Ther. 2014, 18, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Chiewitz, O.; Hevesy, G. Radioactive Indicators in the Study of Phosphorus Metabolism in Rats. Nature 1935, 136, 754–755. [Google Scholar] [CrossRef]
- Fleming, W.H.; McIlraith, J.D.; King, E.R. Photoscanning of Bone Lesions Utilizing Strontium 85. Radiology 1961, 77, 635–636. [Google Scholar] [CrossRef]
- Subramanian, G.; McAfee, J.G. A New Complex of 99mTc for Skeletal Imaging. Radiology 1971, 99, 192–196. [Google Scholar] [CrossRef]
- Blake, G.M.; Park-Holohan, S.J.; Cook, G.J.; Fogelman, I. Quantitative Studies of Bone with the Use of 18F-Fluoride and 99mTc-Methylene Diphosphonate. Semin. Nucl. Med. 2001, 31, 28–49. [Google Scholar] [CrossRef]
- Fleisch, H.; Russell, R.G.; Bisaz, S.; Casey, P.A.; Mühlbauer, R.C. The Influence of Pyrophosphate Analogues (Diphosphonates) on the Precipitation and Dissolution. Calcif. Tissue Res. 1968, 2, 10. [Google Scholar] [CrossRef]
- Lin, J.H. Bisphosphonates: A Review of Their Pharmacokinetic Properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef]
- Budán, F.; Szigeti, K.; Weszl, M.; Horváth, I.; Balogh, E.; Kanaan, R.; Berényi, K.; Lacza, Z.; Máthé, D.; Gyöngyi, Z. Novel Radiomics Evaluation of Bone Formation Utilizing Multimodal (SPECT/X-Ray CT) in Vivo Imaging. PLoS ONE 2018, 13, e0204423. [Google Scholar] [CrossRef]
- Ahuja, K.; Sotoudeh, H.; Galgano, S.J.; Singh, R.; Gupta, N.; Gaddamanugu, S.; Choudhary, G. 18F-Sodium Fluoride PET: History, Technical Feasibility, Mechanism of Action, Normal Biodistribution, and Diagnostic Performance in Bone Metastasis Detection Compared with Other Imaging Modalities. J. Nucl. Med. Technol. 2020, 48, 9–16. [Google Scholar] [CrossRef]
- Hosking, D.J.; Chamberlain, M.J. Studies in Man with 18 F. Clin. Sci. 1972, 42, 153–161. [Google Scholar] [CrossRef]
- Whitford, G.M. Intake and Metabolism of Fluoride. Adv. Dent. Res. 1994, 8, 5–14. [Google Scholar] [CrossRef]
- Aspenberg, P.; Tägil, M.; Kristensson, C.; Lidin, S. Bone Graft Proteins Influence Osteoconduction. A Titanium Chamber Study in Rats. Acta Orthop. Scand. 1996, 67, 377–382. [Google Scholar] [CrossRef]
- Sasaki, H.; Koyama, S.; Yokoyama, M.; Yamaguchi, K.; Itoh, M.; Sasaki, K. Bone Metabolic Activity around Dental Implants under Loading Observed Using Bone Scintigraphy. Int. J. Oral Maxillofac. Implants 2008, 23, 827–834. [Google Scholar]
- McCracken, M.; Zinn, K.; Lemons, J.E.; Thompson, J.A.; Feldman, D. Radioimaging of Implants in Rats Using Tc-99m-MDP. Clin. Oral Implants Res. 2001, 12, 372–378. [Google Scholar] [CrossRef]
- Polyak, A.; Haasz, V.; Postenyi, Z.; Ivanovska, A.; Ross, T.L.; Mathe, D.; Balogh, L. Imaging of Stem Cell Differentiated Bone Implantations with [Tc-99m] Tc-MDP. J. Label. Compd. Radiopharm. 2017, 60, S275. [Google Scholar]
- Rennen, H.J.J.M.; Boerman, O.C.; Oyen, W.J.G.; Corstens, F.H.M. Imaging Infection/Inflammation in the New Millennium. Eur. J. Nucl. Med. 2001, 28, 241–252. [Google Scholar] [CrossRef]
- Thakur, M.L.; Coleman, R.E.; Welch, M.J. Indium-111-Labeled Leukocytes for the Localization of Abscesses: Preparation, Analysis, Tissue Distribution, and Comparison with Gallium-67 Citrate in Dogs. J. Lab. Clin. Med. 1977, 89, 217–228. [Google Scholar] [PubMed]
- Signore, A.; Jamar, F.; Israel, O.; Buscombe, J.; Martin-Comin, J.; Lazzeri, E. Clinical Indications, Image Acquisition and Data Interpretation for White Blood Cells and Anti-Granulocyte Monoclonal Antibody Scintigraphy: An EANM Procedural Guideline. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1816–1831. [Google Scholar] [CrossRef]
- Palestro, C.J.; Kim, C.K.; Swyer, A.J.; Capozzi, J.D.; Solomon, R.W.; Goldsmith, S.J. Total-Hip Arthroplasty: Periprosthetic Indium-111-Labeled Leukocyte Activity and Complementary Technetium-99m-Sulfur Colloid Imaging in Suspected Infection. J. Nucl. Med. 1990, 31, 1950–1955. [Google Scholar]
- Liberatore, M.; Iurilli, A.P.; Ponzo, F.; Prosperi, D.; Santini, C.; Baiocchi, P.; Rizzo, L.; Speziale, F.; Fiorani, P.; Colella, A.C. Clinical Usefulness of Technetium-99m-HMPAO-Labeled Leukocyte Scan in Prosthetic Vascular Graft Infection. J. Nucl. Med. 1998, 39, 875–879. [Google Scholar] [PubMed]
- Erba, P.A.; Conti, U.; Lazzeri, E.; Sollini, M.; Doria, R.; De Tommasi, S.M.; Bandera, F.; Tascini, C.; Menichetti, F.; Dierckx, R.A.J.O.; et al. Added Value of 99mTc-HMPAO-Labeled Leukocyte SPECT/CT in the Characterization and Management of Patients with Infectious Endocarditis. J. Nucl. Med. 2012, 53, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Litzler, P.-Y.; Manrique, A.; Etienne, M.; Salles, A.; Edet-Sanson, A.; Vera, P.; Bessou, J.-P.; Hitzel, A. Leukocyte SPECT/CT for Detecting Infection of Left-Ventricular-Assist Devices: Preliminary Results. J. Nucl. Med. 2010, 51, 1044–1048. [Google Scholar] [CrossRef]
- Bartel, T.B.; Kuruva, M.; Gnanasegaran, G.; Beheshti, M.; Cohen, E.J.; Weissman, A.F.; Yarbrough, T.L. SNMMI Procedure Standard for Bone Scintigraphy 4.0. J. Nucl. Med. Technol. 2018, 46, 398–404. [Google Scholar]
- Love, C.; Tomas, M.B.; Tronco, G.G.; Palestro, C.J. FDG PET of Infection and Inflammation. Radiographics 2005, 25, 1357–1368. [Google Scholar] [CrossRef]
- Shemesh, S.; Kosashvili, Y.; Groshar, D.; Bernstine, H.; Sidon, E.; Cohen, N.; Luria, T.; Velkes, S. The Value of 18-FDG PET/CT in the Diagnosis and Management of Implant-Related Infections of the Tibia: A Case Series. Injury 2015, 46, 1377–1382. [Google Scholar] [CrossRef]
- Wenter, V.; Müller, J.-P.; Albert, N.L.; Lehner, S.; Fendler, W.P.; Bartenstein, P.; Cyran, C.C.; Friederichs, J.; Militz, M.; Hacker, M.; et al. The Diagnostic Value of [18F]FDG PET for the Detection of Chronic Osteomyelitis and Implant-Associated Infection. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 749–761. [Google Scholar] [CrossRef]
- Bhoil, A.; Caw, H.; Vinjamuri, S. Role of 18F-Flurodeoxyglucose in Orthopaedic Implant-Related Infection: Review of Literature and Experience. Nucl. Med. Commun. 2019, 40, 875–887. [Google Scholar] [CrossRef]
- Kouijzer, I.J.E.; Scheper, H.; de Rooy, J.W.J.; Bloem, J.L.; Janssen, M.J.R.; van den Hoven, L.; Hosman, A.J.F.; Visser, L.G.; Oyen, W.J.G.; Bleeker-Rovers, C.P.; et al. The Diagnostic Value of 18F–FDG-PET/CT and MRI in Suspected Vertebral Osteomyelitis—A Prospective Study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 798–805. [Google Scholar] [CrossRef]
- Basu, S.; Kwee, T.C.; Saboury, B.; Garino, J.P.; Nelson, C.L.; Zhuang, H.; Parsons, M.; Chen, W.; Kumar, R.; Salavati, A.; et al. FDG PET for Diagnosing Infection in Hip and Knee Prostheses: Prospective Study in 221 Prostheses and Subgroup Comparison with Combined (111)In-Labeled Leukocyte/(99m)Tc-Sulfur Colloid Bone Marrow Imaging in 88 Prostheses. Clin. Nucl. Med. 2014, 39, 609–615. [Google Scholar] [CrossRef]
- Juneau, D.; Golfam, M.; Hazra, S.; Zuckier, L.S.; Garas, S.; Redpath, C.; Bernick, J.; Leung, E.; Chih, S.; Wells, G.; et al. Positron Emission Tomography and Single-Photon Emission Computed Tomography Imaging in the Diagnosis of Cardiac Implantable Electronic Device Infection: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2017, 10, e005772. [Google Scholar] [CrossRef]
- Abgral, R.; Dweck, M.R.; Trivieri, M.G.; Robson, P.M.; Karakatsanis, N.; Mani, V.; Padilla, M.; Miller, M.; Lala, A.; Sanz, J.; et al. Clinical Utility of Combined FDG-PET/MR to Assess Myocardial Disease. JACC Cardiovasc. Imaging 2017, 10, 594–597. [Google Scholar] [CrossRef]
- Wada, K.; Niitsuma, T.; Yamaki, T.; Masuda, A.; Ito, H.; Kubo, H.; Hara, T.; Takenoshita, S.; Takeishi, Y. Simultaneous Cardiac Imaging to Detect Inflammation and Scar Tissue with (18)F-Fluorodeoxyglucose PET/MRI in Cardiac Sarcoidosis. J. Nucl. Cardiol. 2016, 23, 1180–1182. [Google Scholar] [CrossRef]
- Einspieler, I.; Thürmel, K.; Pyka, T.; Eiber, M.; Wolfram, S.; Moog, P.; Reeps, C.; Essler, M. Imaging Large Vessel Vasculitis with Fully Integrated PET/MRI: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1012–1024. [Google Scholar] [CrossRef]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.J.A.; Lindner, O.; Sciagra, R.; Agostini, D.; Übleis, C.; Gimelli, A.; Hacker, M.; et al. Position Paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET Imaging of Atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef]
- Hyafil, F.; Pelisek, J.; Laitinen, I.; Schottelius, M.; Mohring, M.; Döring, Y.; van der Vorst, E.P.C.; Kallmayer, M.; Steiger, K.; Poschenrieder, A.; et al. Imaging the Cytokine Receptor CXCR4 in Atherosclerotic Plaques with the Radiotracer 68Ga-Pentixafor for PET. J. Nucl. Med. 2017, 58, 499–506. [Google Scholar] [CrossRef]
- Gourni, E.; Demmer, O.; Schottelius, M.; D’Alessandria, C.; Schulz, S.; Dijkgraaf, I.; Schumacher, U.; Schwaiger, M.; Kessler, H.; Wester, H.-J. PET of CXCR4 Expression by a 68Ga-Labeled Highly Specific Targeted Contrast Agent. J. Nucl. Med. 2011, 52, 1803–1810. [Google Scholar] [CrossRef]
- Döring, Y.; Pawig, L.; Weber, C.; Noels, H. The CXCL12/CXCR4 Chemokine Ligand/Receptor Axis in Cardiovascular Disease. Front. Physiol. 2014, 5, 212. [Google Scholar] [CrossRef]
- Toczek, J.; Riou, L. Considerations on PET/MR Imaging of Carotid Plaque Inflammation with 68Ga-Pentixafor. J. Nucl. Cardiol. 2022, 29, 503–505. [Google Scholar] [CrossRef]
- Li, X.; Heber, D.; Leike, T.; Beitzke, D.; Lu, X.; Zhang, X.; Wei, Y.; Mitterhauser, M.; Wadsak, W.; Kropf, S.; et al. [68Ga]Pentixafor-PET/MRI for the Detection of Chemokine Receptor 4 Expression in Atherosclerotic Plaques. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 558–566. [Google Scholar] [CrossRef]
- Kircher, M.; Tran-Gia, J.; Kemmer, L.; Zhang, X.; Schirbel, A.; Werner, R.A.; Buck, A.K.; Wester, H.-J.; Hacker, M.; Lapa, C.; et al. Imaging Inflammation in Atherosclerosis with CXCR4-Directed 68Ga-Pentixafor PET/CT: Correlation with 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Calabretta, R.; Wadsak, W.; Haug, A.R.; Mayerhöfer, M.; Raderer, M.; Zhang, X.; Li, J.; Hacker, M.; Li, X. Imaging Inflammation in Atherosclerosis with CXCR4-Directed [68Ga]PentixaFor PET/MRI—Compared with [18F]FDG PET/MRI. Life 2022, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.O.; Popoola, G.O.; Mahapane, J.; Kaufmann, J.; Davis, C.; Ndlovu, H.; Maserumule, L.C.; Mokoala, K.M.G.; Bouterfa, H.; Wester, H.-J.; et al. [68Ga]Ga-Pentixafor for PET Imaging of Vascular Expression of CXCR-4 as a Marker of Arterial Inflammation in HIV-Infected Patients: A Comparison with 18F[FDG] PET Imaging. Biomolecules 2020, 10, 1629. [Google Scholar] [CrossRef] [PubMed]
- Bouter, C.; Meller, B.; Sahlmann, C.O.; Staab, W.; Wester, H.J.; Kropf, S.; Meller, J. 68Ga-Pentixafor PET/CT Imaging of Chemokine Receptor CXCR4 in Chronic Infection of the Bone: First Insights. J. Nucl. Med. 2018, 59, 320–326. [Google Scholar] [CrossRef]
- Buchert, R.; Dirks, M.; Schütze, C.; Wilke, F.; Mamach, M.; Wirries, A.-K.; Pflugrad, H.; Hamann, L.; Langer, L.B.N.; Wetzel, C.; et al. Reliable Quantification of 18F-GE-180 PET Neuroinflammation Studies Using an Individually Scaled Population-Based Input Function or Late Tissue-to-Blood Ratio. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2887–2900. [Google Scholar] [CrossRef]
- Hermanns, N.; Wroblewski, V.; Bascuñana, P.; Wolf, B.; Polyak, A.; Ross, T.L.; Bengel, F.M.; Thackeray, J.T. Molecular Imaging of the Brain–Heart Axis Provides Insights into Cardiac Dysfunction after Cerebral Ischemia. Basic Res. Cardiol. 2022, 117, 52. [Google Scholar] [CrossRef]
- Gowrishankar, G.; Namavari, M.; Jouannot, E.B.; Hoehne, A.; Reeves, R.; Hardy, J.; Gambhir, S.S. Investigation of 6-[18F]-Fluoromaltose as a Novel PET Tracer for Imaging Bacterial Infection. PLoS ONE 2014, 9, e107951. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Houson, H.A.; Kamble, N.S.; Blanco, J.R.; O’Donnell, R.E.; Hassett, D.J.; Lapi, S.E.; Kotagiri, N. Leveraging Copper Import by Yersiniabactin Siderophore System for Targeted PET Imaging of Bacteria. JCI Insight 2021, 6, e144880. [Google Scholar] [CrossRef]
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell Surface Glycoprotein of Reactive Stromal Fibroblasts as a Potential Antibody Target in Human Epithelial Cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding Fibroblast Activation Protein (FAP): Substrates, Activities, Expression and Targeting for Cancer Therapy. Proteom.–Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.B.N.; Hess, A.; Korkmaz, Z.; Tillmanns, J.; Reffert, L.M.; Bankstahl, J.P.; Bengel, F.M.; Thackeray, J.T.; Ross, T.L. Molecular Imaging of Fibroblast Activation Protein after Myocardial Infarction Using the Novel Radiotracer [(68)Ga]MHLL1. Theranostics 2021, 11, 7755–7766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, X.; Liu, H.; Luo, W.; Liu, H.; Lv, T.; Wang, J.; Qin, J.; Ou, S.; Chen, Y. Value of [(68)Ga]Ga-FAPI-04 Imaging in the Diagnosis of Renal Fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, J.; Fu, K.; Lin, Q.; Chen, H. 68Ga-FAPI PET/CT in Assessment of Liver Nodules in a Cirrhotic Patient. Clin. Nucl. Med. 2020, 45, e430–e432. [Google Scholar] [CrossRef]
- Guo, W.; Pang, Y.; Yao, L.; Zhao, L.; Fan, C.; Ke, J.; Guo, P.; Hao, B.; Fu, H.; Xie, C. Imaging Fibroblast Activation Protein in Liver Cancer: A Single-Center Post Hoc Retrospective Analysis to Compare [68Ga] Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1604–1617. [Google Scholar] [CrossRef]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Stem Cell Therapy. Use of Differentiated Pluripotent Stem Cells as Replacement Therapy for Treating Disease. Science 2014, 345, 1247391. [Google Scholar] [CrossRef]
- Wang, J.; Jokerst, J.V. Stem Cell Imaging: Tools to Improve Cell Delivery and Viability. Stem Cells Int. 2016, 2016, 9240652. [Google Scholar] [CrossRef]
- Dudhia, J.; Becerra, P.; Valdés, M.A.; Neves, F.; Hartman, N.G.; Smith, R.K.W. In Vivo Imaging and Tracking of Technetium-99m Labeled Bone Marrow Mesenchymal Stem Cells in Equine Tendinopathy. J. Vis. Exp. 2015, 106, e52748. [Google Scholar] [CrossRef]
- Welch, M.J.; Thakur, M.L.; Coleman, R.E.; Patel, M.; Siegel, B.A.; Ter-Pogossian, M. Gallium-68 Labeled Red Cells and Platelets: New Agents for Positron Tomography. J. Nucl. Med. 1977, 18, 558–562. [Google Scholar]
- Forstrom, L.A.; Mullan, B.P.; Hung, J.C.; Lowe, V.J.; Thorson, L.M. 18F-FDG Labelling of Human Leukocytes. Nucl. Med. Commun. 2000, 21, 685–690. [Google Scholar] [CrossRef]
- Stojanov, K.; de Vries, E.F.J.; Hoekstra, D.; van Waarde, A.; Dierckx, R.A.J.O.; Zuhorn, I.S. [18F]FDG Labeling of Neural Stem Cells for in Vivo Cell Tracking with Positron Emission Tomography: Inhibition of Tracer Release by Phloretin. Mol. Imaging 2012, 11, 1–12. [Google Scholar] [CrossRef]
- Elhami, E.; Goertzen, A.L.; Xiang, B.; Deng, J.; Stillwell, C.; Mzengeza, S.; Arora, R.C.; Freed, D.; Tian, G. Viability and Proliferation Potential of Adipose-Derived Stem Cells Following Labeling with a Positron-Emitting Radiotracer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1323–1334. [Google Scholar] [CrossRef]
- Adonai, N.; Nguyen, K.N.; Walsh, J.; Iyer, M.; Toyokuni, T.; Phelps, M.E.; McCarthy, T.; McCarthy, D.W.; Gambhir, S.S. Ex Vivo Cell Labeling with 64Cu–Pyruvaldehyde-Bis(N4-Methylthiosemicarbazone) for Imaging Cell Trafficking in Mice with Positron-Emission Tomography. Proc. Natl. Acad. Sci. USA 2002, 99, 3030–3035. [Google Scholar] [CrossRef]
- Huang, J.; Lee, C.C.I.; Sutcliffe, J.L.; Cherry, S.R.; Tarantal, A.F. Radiolabeling Rhesus Monkey CD34+ Hematopoietic and Mesenchymal Stem Cells with 64Cu-Pyruvaldehyde-Bis(N4-Methylthiosemicarbazone) for MicroPET Imaging. Mol. Imaging 2008, 7, 1–11. [Google Scholar] [CrossRef]
- Kim, M.H.; Woo, S.-K.; Kim, K.I.; Lee, T.S.; Kim, C.W.; Kang, J.H.; Kim, B., II; Lim, S.M.; Lee, K.C.; Lee, Y.J. Simple Methods for Tracking Stem Cells with 64Cu-Labeled DOTA-Hexadecyl-Benzoate. ACS Med. Chem. Lett. 2015, 6, 528–530. [Google Scholar] [CrossRef]
- Patrick, P.S.; Kolluri, K.K.; Thin, M.Z.; Edwards, A.; Sage, E.K.; Sanderson, T.; Weil, B.D.; Dickson, J.C.; Lythgoe, M.F.; Lowdell, M.; et al. 89Zr-Oxine Labelling and PET Imaging Shows Lung Delivery of a Cell/Gene Cancer Therapy. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ferris, T.J.; Charoenphun, P.; Meszaros, L.K.; Mullen, G.E.D.; Blower, P.J.; Went, M.J. Synthesis and Characterisation of Zirconium Complexes for Cell Tracking with Zr-89 by Positron Emission Tomography. Dalt. Trans. 2014, 43, 14851–14857. [Google Scholar] [CrossRef]
- Charoenphun, P.; Meszaros, L.K.; Chuamsaamarkkee, K.; Sharif-Paghaleh, E.; Ballinger, J.R.; Ferris, T.J.; Went, M.J.; Mullen, G.E.D.; Blower, P.J. [89Zr]Oxinate4 for Long-Term in Vivo Cell Tracking by Positron Emission Tomography. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 278–287. [Google Scholar] [CrossRef]
- Polyak, A.; Bankstahl, J.P.; Besecke, K.F.W.; Hozsa, C.; Triebert, W.; Pannem, R.R.; Manstein, F.; Borcholte, T.; Furch, M.; Zweigerdt, R.; et al. Simplified 89Zr-Labeling Protocol of Oxine (8-Hydroxyquinoline) Enabling Prolonged Tracking of Liposome-Based Nanomedicines and Cells. Pharmaceutics 2021, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Lim, L.; Volpe, A.; Gabizon, A.; Shmeeda, H.; Draper, B.; Parente-Pereira, A.C.; Maher, J.; Blower, P.J.; Fruhwirth, G.O.; et al. In Vivo PET Tracking of (89)Zr-Labeled Vγ9Vδ2 T Cells to Mouse Xenograft Breast Tumors Activated with Liposomal Alendronate. Mol. Ther. 2019, 27, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.S.; Kolluri, K.K.; Zaw Thin, M.; Edwards, A.; Sage, E.K.; Sanderson, T.; Weil, B.D.; Dickson, J.C.; Lythgoe, M.F.; Lowdell, M.; et al. Lung Delivery of MSCs Expressing Anti-Cancer Protein TRAIL Visualised with 89Zr-Oxine PET-CT. Stem Cell Res. Ther. 2020, 11, 256. [Google Scholar] [CrossRef]
- Jauw, Y.W.S.; Menke-van der Houven van Oordt, C.W.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; van Dongen, G.A.M.S. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front. Pharmacol. 2016, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Wu, H.; Asiedu, K.O.; Szajek, L.P.; Griffiths, G.L.; Choyke, P.L. (89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-Based Therapies. Radiology 2015, 275, 490–500. [Google Scholar] [CrossRef]
- Asiedu, K.O.; Koyasu, S.; Szajek, L.P.; Choyke, P.L.; Sato, N. Bone Marrow Cell Trafficking Analyzed by 89Zr-Oxine Positron Emission Tomography in a Murine Transplantation Model. Clin. Cancer Res. 2017, 23, 2759–2768. [Google Scholar] [CrossRef]
- Asiedu, K.O.; Ferdousi, M.; Ton, P.T.; Adler, S.S.; Choyke, P.L.; Sato, N. Bone Marrow Cell Homing to Sites of Acute Tibial Fracture: 89Zr-Oxine Cell Labeling with Positron Emission Tomographic Imaging in a Mouse Model. EJNMMI Res. 2018, 8, 109. [Google Scholar] [CrossRef]
- Weist, M.R.; Starr, R.; Aguilar, B.; Chea, J.; Miles, J.K.; Poku, E.; Gerdts, E.; Yang, X.; Priceman, S.J.; Forman, S.J.; et al. PET of Adoptively Transferred Chimeric Antigen Receptor T Cells with (89)Zr-Oxine. J. Nucl. Med. 2018, 59, 1531–1537. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic Iron Oxide Nanoparticles for Drug Delivery: Applications and Characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Sarko, D.; Eisenhut, M.; Haberkorn, U.; Mier, W. Bifunctional Chelators in the Design and Application of Radiopharmaceuticals for Oncological Diseases. Curr. Med. Chem. 2012, 19, 2667–2688. [Google Scholar] [CrossRef]
- Spang, P.; Herrmann, C.; Roesch, F. Bifunctional Gallium-68 Chelators: Past, Present, and Future. Semin. Nucl. Med. 2016, 46, 373–394. [Google Scholar] [CrossRef]
- Lewis, M.R.; Wang, M.; Axworthy, D.B.; Theodore, L.J.; Mallet, R.W.; Fritzberg, A.R.; Welch, M.J.; Anderson, C.J. In Vivo Evaluation of Pretargeted 64Cu for Tumor Imaging and Therapy. J. Nucl. Med. 2003, 44, 1284–1292. [Google Scholar]
- Cheal, S.M.; Chung, S.K.; Vaughn, B.A.; Cheung, N.-K.V.; Larson, S.M. Pretargeting: A Path Forward for Radioimmunotherapy. J. Nucl. Med. 2022, 63, 1302–1315. [Google Scholar] [CrossRef]
- van de Watering, F.C.J.; Rijpkema, M.; Robillard, M.; Oyen, W.J.G.; Boerman, O.C. Pretargeted Imaging and Radioimmunotherapy of Cancer Using Antibodies and Bioorthogonal Chemistry. Front. Med. 2014, 1, 44. [Google Scholar] [CrossRef]
- Orcutt, K.D.; Slusarczyk, A.L.; Cieslewicz, M.; Ruiz-Yi, B.; Bhushan, K.R.; Frangioni, J.V.; Wittrup, K.D. Engineering an Antibody with Picomolar Affinity to DOTA Chelates of Multiple Radionuclides for Pretargeted Radioimmunotherapy and Imaging. Nucl. Med. Biol. 2011, 38, 223–233. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Kaul, S.; Klivényi, G.; Junkermann, H.; Magener, A.; Henze, M.; Doll, J.; Haberkorn, U.; Amelung, F.; Bastert, G. Immunoscintigraphy with Positron Emission Tomography: Gallium-68 Chelate Imaging of Breast Cancer Pretargeted with Bispecific Anti-MUC1/Anti-Ga Chelate Antibodies. Cancer Res. 2001, 61, 3712–3717. [Google Scholar]
- Bodet-Milin, C.; Faivre-Chauvet, A.; Carlier, T.; Rauscher, A.; Bourgeois, M.; Cerato, E.; Rohmer, V.; Couturier, O.; Drui, D.; Goldenberg, D.M.; et al. Immuno-PET Using Anticarcinoembryonic Antigen Bispecific Antibody and 68Ga-Labeled Peptide in Metastatic Medullary Thyroid Carcinoma: Clinical Optimization of the Pretargeting Parameters in a First-in-Human Trial. J. Nucl. Med. 2016, 57, 1505–1511. [Google Scholar] [CrossRef]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; van Herpen, C.M.L.; Franssen, G.M.; McBride, W.J.; Chang, C.-H.; Rossi, E.A.; van der Graaf, W.T.A.; et al. Development of an Imaging-Guided CEA-Pretargeted Radionuclide Treatment of Advanced Colorectal Cancer: First Clinical Results. Br. J. Cancer 2013, 109, 934–942. [Google Scholar] [CrossRef]
- Rousseau, C.; Goldenberg, D.M.; Colombié, M.; Sébille, J.-C.; Meingan, P.; Ferrer, L.; Baumgartner, P.; Cerato, E.; Masson, D.; Campone, M.; et al. Initial Clinical Results of a Novel Immuno-PET Theranostic Probe in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer. J. Nucl. Med. 2020, 61, 1205–1211. [Google Scholar] [CrossRef]
- Kraeber-Bodéré, F.; Salaun, P.-Y.; Ansquer, C.; Drui, D.; Mirallié, E.; Faivre-Chauvet, A.; Barbet, J.; Goldenberg, D.M.; Chatal, J.-F. Pretargeted Radioimmunotherapy (PRAIT) in Medullary Thyroid Cancer (MTC). Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2012, 33, 601–606. [Google Scholar] [CrossRef]
- Salaun, P.-Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.-P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase II Trial of Anticarcinoembryonic Antigen Pretargeted Radioimmunotherapy in Progressive Metastatic Medullary Thyroid Carcinoma: Biomarker Response and Survival Improvement. J. Nucl. Med. 2012, 53, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- de Boisferon, M.H.; Manetti, C.; Raguin, O.; Gautherot, E.; Rostène, W.; Barbet, J.; Gruaz-Guyon, A. Pretargeted Radioimmunotherapy Using131I-Labelled Bivalent Hapten-Bearing Peptides. Lett. Pept. Sci. 1997, 4, 331–339. [Google Scholar] [CrossRef]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.; van Osdol, W.W. Pharmacokinetic Comparison of Direct Antibody Targeting with Pretargeting Protocols Based on Streptavidin-Biotin Binding. J. Nucl. Med. 1995, 36, 867–876. [Google Scholar]
- Casalini, P.; Luison, E.; Ménard, S.; Colnaghi, M.I.; Paganelli, G.; Canevari, S. Tumor Pretargeting: Role of Avidin/Streptavidin on Monoclonal Antibody Internalization. J. Nucl. Med. 1997, 38, 1378–1381. [Google Scholar]
- Kalofonos, H.P.; Rusckowski, M.; Siebecker, D.A.; Sivolapenko, G.B.; Snook, D.; Lavender, J.P.; Epenetos, A.A.; Hnatowich, D.J. Imaging of Tumor in Patients with Indium-111-Labeled Biotin and Streptavidin-Conjugated Antibodies: Preliminary Communication. J. Nucl. Med. 1990, 31, 1791–1796. [Google Scholar]
- Liu, G.; Mang’era, K.; Liu, N.; Gupta, S.; Rusckowski, M.; Hnatowich, D.J. Tumor Pretargeting in Mice Using 99mTc-Labeled Morpholino, a DNA Analog. J. Nucl. Med. 2002, 43, 384–391. [Google Scholar]
- Liu, G.; Dou, S.; Liu, Y.; Wang, Y.; Rusckowski, M.; Hnatowich, D.J. 90Y Labeled Phosphorodiamidate Morpholino Oligomer for Pretargeting Radiotherapy. Bioconjug. Chem. 2011, 22, 2539–2545. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Dou, S.; Liu, X.; Zhang, S.; Liu, G.; Hnatowich, D. Affinity Enhancement Pretargeting: Synthesis and Testing of a 99mTc-Labeled Bivalent MORF. Mol. Pharm. 2010, 7, 1118–1124. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Mohindra, P.; Weissmann, G.I.; Divilov, V.; Hilderbrand, S.A.; Weissleder, R.; Lewis, J.S. Modular Strategy for the Construction of Radiometalated Antibodies for Positron Emission Tomography Based on Inverse Electron Demand Diels–Alder Click Chemistry. Bioconjug. Chem. 2011, 22, 2048–2059. [Google Scholar] [CrossRef]
- Baranyai, Z.; Reich, D.; Vágner, A.; Weineisen, M.; Tóth, I.; Wester, H.-J.; Notni, J. A Shortcut to High-Affinity Ga-68 and Cu-64 Radiopharmaceuticals: One-Pot Click Chemistry Trimerisation on the TRAP Platform. Dalt. Trans. 2015, 44, 11137–11146. [Google Scholar] [CrossRef]
- García, M.F.; Zhang, X.; Shah, M.; Newton-Northup, J.; Cabral, P.; Cerecetto, H.; Quinn, T. 99mTc-Bioorthogonal Click Chemistry Reagent for in Vivo Pretargeted Imaging. Bioorg. Med. Chem. 2016, 24, 1209–1215. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Lin, W.-Y.; Shen, C.K.-F.; Yap, L.-P.; Hughes, L.D.; Conti, P.S. Strain-Promoted Catalyst-Free Click Chemistry for Rapid Construction of 64Cu-Labeled PET Imaging Probes. ACS Med. Chem. Lett. 2012, 3, 1019–1023. [Google Scholar] [CrossRef]
- Das, T.; Pillai, M.R.A. Options to Meet the Future Global Demand of Radionuclides for Radionuclide Therapy. Nucl. Med. Biol. 2013, 40, 23–32. [Google Scholar] [CrossRef]
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A Roadmap for Future Development. Lancet. Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hänscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. [(177)Lu]Pentixather: Comprehensive Preclinical Characterization of a First CXCR4-Directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef]
- Habringer, S.; Lapa, C.; Herhaus, P.; Schottelius, M.; Istvanffy, R.; Steiger, K.; Slotta-Huspenina, J.; Schirbel, A.; Hänscheid, H.; Kircher, S.; et al. Dual Targeting of Acute Leukemia and Supporting Niche by CXCR4-Directed Theranostics. Theranostics 2018, 8, 369–383. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef]
- Lapa, C.; Herrmann, K.; Schirbel, A.; Hänscheid, H.; Lückerath, K.; Schottelius, M.; Kircher, M.; Werner, R.A.; Schreder, M.; Samnick, S.; et al. CXCR4-Directed Endoradiotherapy Induces High Response Rates in Extramedullary Relapsed Multiple Myeloma. Theranostics 2017, 7, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Pang, Y.; Zhao, L.; Lin, L.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. FAP-Targeted Radionuclide Therapy with [(177)Lu]Lu-FAPI-46 in Metastatic Nasopharyngeal Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Rathke, H.; Fink, R.; Dendl, K.; Debus, J.; Mier, W.; Jäger, D.; Lindner, T.; Haberkorn, U. [(153)Sm]Samarium-Labeled FAPI-46 Radioligand Therapy in a Patient with Lung Metastases of a Sarcoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3011–3013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast Activation Protein Targeted Therapy Using [(177)Lu]FAPI-46 Compared with [(225)Ac]FAPI-46 in a Pancreatic Cancer Model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 871–880. [Google Scholar] [CrossRef]
- Peters, S.M.B.; Privé, B.M.; de Bakker, M.; de Lange, F.; Jentzen, W.; Eek, A.; Muselaers, C.H.J.; Mehra, N.; Witjes, J.A.; Gotthardt, M.; et al. Intra-Therapeutic Dosimetry of [(177)Lu]Lu-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer Patients and Correlation with Treatment Outcome. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 460–469. [Google Scholar] [CrossRef]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using (177)Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
- Privé, B.M.; Boussihmad, M.A.; Timmermans, B.; van Gemert, W.A.; Peters, S.M.B.; Derks, Y.H.W.; van Lith, S.A.M.; Mehra, N.; Nagarajah, J.; Heskamp, S.; et al. Fibroblast Activation Protein-Targeted Radionuclide Therapy: Background, Opportunities, and Challenges of First (Pre)Clinical Studies. Eur. J. Nucl. Med. Mol. Imaging 2023, 1–13. [Google Scholar] [CrossRef]




| Labeled Compound (Modality) | Mechanism | Promise | Limitations |
|---|---|---|---|
| 2-[18F]FDG (PET) | indicates any enhanced tissue glucose metabolism | infection, inflammation | lower specificity, uptake in heart and brain, blood background signal, interaction with blood glucose |
| Na[18F]F (PET) | indicates enhanced bone metabolism | osseointegration, bone regeneration, bone resorption | lower specificity, lower imaging sensitivity compared to alternatives |
| [68Ga]FAPI (PET) | indicates fibrosis by targeting fibroblast activation protein (FAP) | inflammation, fibrosis, wound healing | limited specificity, inter-subject variability, limited availability, higher costs |
| [18F]GE180 (PET) | indicates inflammatory by translocator protein (TSPO) | inflammation | |
| 68Ga-pentixafor (PET) | indicates C-X-C chemokine receptor type 4 (CXCR4) | inflammation | |
| 18F-maltohexaose (PET) | specific for bacteria, indicates infections, allows to distinguish bacterial infections from sterile inflammations | infection, biofilms | limited specificity, antibiotics may preclude its application |
| 111In-oxine-leukocytes (SPECT) | indicates infections and sterile inflammations by labeling of white blood cells | infection, inflammation | lower specificity, partly invasive |
| [99mTc]Tc-HMPAO labeled and 111In-, 68Ga-, 89Zr-oxine labeled cells (SPECT or PET) | lipophilic prelabeled agent internalized by the cells | cell tracking | cell viability and function must be considered |
| 99mTc-labeled phosphonates, e.g., MDP (SPECT) | indicates enhanced bone metabolism | osseointegration, bone regeneration, bone resorption, infection | limited specificity, inflammation, infection, or healing fractures can increase the uptake |
| 18F-, 68Ga-, 89Zr-, 64Cu-, 99mTc-labeled nanoparticles (SPECT or PET) | direct tracking of nanocarriers | real-time tracking, evaluation of NP pharmacokinetics and biodistribution, imaging guided drug delivery, personalized medicine | limited tracing time depending on the selected radionuclide |
| pretargeted ligand, prelabeled agents (SPECT or PET) | selective pretargeting, click chemistry | specific imaging of preselected ligand, target | unique reactions, advanced bio- and radiochemistry |
| radiotheranostic agents (RNT, RT, ERT, theranosis, SPECT, PET) | switchable radioisotopes | imaging guided therapy, personalized medicine | availability, higher cost, advanced radiochemistry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyak, A.; Képes, Z.; Trencsényi, G. Implant Imaging: Perspectives of Nuclear Imaging in Implant, Biomaterial, and Stem Cell Research. Bioengineering 2023, 10, 521. https://doi.org/10.3390/bioengineering10050521
Polyak A, Képes Z, Trencsényi G. Implant Imaging: Perspectives of Nuclear Imaging in Implant, Biomaterial, and Stem Cell Research. Bioengineering. 2023; 10(5):521. https://doi.org/10.3390/bioengineering10050521
Chicago/Turabian StylePolyak, Andras, Zita Képes, and György Trencsényi. 2023. "Implant Imaging: Perspectives of Nuclear Imaging in Implant, Biomaterial, and Stem Cell Research" Bioengineering 10, no. 5: 521. https://doi.org/10.3390/bioengineering10050521
APA StylePolyak, A., Képes, Z., & Trencsényi, G. (2023). Implant Imaging: Perspectives of Nuclear Imaging in Implant, Biomaterial, and Stem Cell Research. Bioengineering, 10(5), 521. https://doi.org/10.3390/bioengineering10050521