β-TCP/S53P4 Scaffolds Obtained by Gel Casting: Synthesis, Properties, and Biomedical Applications
Abstract
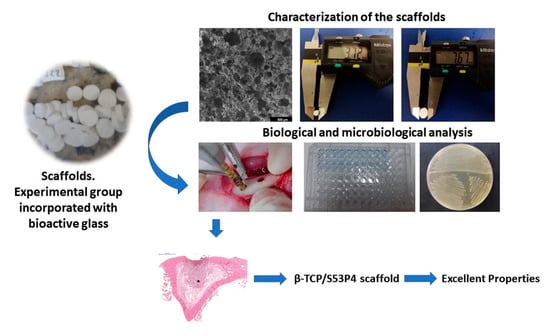
:1. Introduction
2. Materials and Methods
2.1. Fabrication of β-TCP Scaffolds by Gel Casting Method and Incorporation of Bioactive Glass S53P4 by Sol–Gel
2.2. Characterization of β-TCP and β-TCP/S53P4 Scaffolds
2.2.1. X-ray Diffraction (XRD) Analysis
2.2.2. Morphological and Physical Characterization of β-TCP and β-TCP/S53P4 Scaffolds
2.3. Biological Characterization of β-TCP and β-TCP/S53P4 Scaffolds
2.3.1. In Vitro Cell Culture
Cell Morphology
Cell Viability
Total Protein Content
Alkaline Phosphatase (ALP) Assay
Alizarin Red Staining
RNA Extraction and Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
2.3.2. Metabolic Activity of Microorganisms to Assess the Antimicrobial Effect of Scaffolds
2.3.3. Animals and Implantation Procedure
Histological Procedure
Bone Tissue Formation Evaluation by Area Measurement
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillaume, B. Filling bone defects with β-tcp in maxillofacial surgery: A Review. Morphologie 2017, 101, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, S.; Chung, S.H. Clinical outcome of beta-tricalcium phosphate use for bone defects after operative treatment of benign tumors. Clin. Orthop. Surg. 2019, 11, 233–236. [Google Scholar] [CrossRef] [PubMed]
- AL Jasser, R.; AlSubaie, A.; AlShehri, F. Effectiveness of Beta-Tricalcium Phosphate in comparison with other materials in treating periodontal infra-bony defects around natural teeth: A Systematic Review and Meta-Analysis. BMC Oral Health 2021, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Shiota, M.; Yamashita, Y.; Kasugai, S. Histological and histomorphometrical comparative study of the degradation and osteoconductive characteristics of alpha- and beta-tricalcium phosphate in block grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Lemler, J.; Sakurai, K.; Yamada, M. Biodegradation property of beta-tricalcium phosphate-collagen composite in accordance with bone formation: A comparative study with Bio-Oss Collagen® in a rat critical-size defect model. Clin. Implant Dent. Relat. Res. 2014, 16, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yan, D.; Zhou, Q.; Wu, Z.; Weng, S.; Boodhun, V.; Bai, B.; Shen, Z.; Tang, J.; Chen, L.; et al. The Fast Degradation of β-TCP Ceramics facilitates healing of bone defects by the combination of bmp-2 and teriparatide. Biomed. Pharmacother. 2019, 112, 108578. [Google Scholar] [CrossRef]
- Titsinides, S.; Karatzas, T.; Perrea, D.; Eleftheriadis, E.; Podaropoulos, L.; Kalyvas, D.; Katopodis, C.; Agrogiannis, G. Osseous healing in surgically prepared bone defects using different grafting materials: An experimental study in pigs. Dent. J. 2020, 8, 7. [Google Scholar] [CrossRef]
- Hench, L.L. The Story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Jones, J.R. Review of Bioactive Glass: From hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2015, 23 Suppl, S53–S82. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Coraça-Huber, D.C.; Fille, M.; Hausdorfer, J.; Putzer, D.; Nogler, M. Efficacy of antibacterial bioactive glass S53P4 against S. Aureus biofilms grown on titanium discs in vitro. J. Orthop. Res. 2014, 32, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.H.; Magalhães, J.A.; Gouveia, R.F.; Bertran, C.A.; Motisuke, M.; Camargo, S.E.A.; Trichês, E.d.S. Hierarchical structures of β-TCP/45S5 bioglass hybrid scaffolds prepared by gelcasting. J. Mech. Behav. Biomed. Mater. 2016, 62, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Spirandeli, B.R.; Ribas, R.G.; Amaral, S.S.; Martins, E.F.; Esposito, E.; Vasconcellos, L.M.R.; Campos, T.M.B.; Thim, G.P.; Trichês, E.S. Incorporation of 45S5 Bioglass via Sol-Gel in β-TCP Scaffolds: Bioactivity and antimicrobial activity evaluation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 131, 112453. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Närhi, T.O.; Hupa, L. Fiber Glass-Bioactive Glass Composite for bone replacing and bone anchoring implants. Dent. Mater. 2015, 31, 371–381. [Google Scholar] [CrossRef]
- Kastrin, M.; Rovan, V.U.; Frangež, I. Possible advantages of S53P4 bioactive glass in the treatment of septic osteoarthritis of the first metatarsophalangeal joint in the diabetic foot. J. Clin. Med. 2021, 10, 1208. [Google Scholar] [CrossRef]
- Waselau, M.; Patrikoski, M.; Mannerström, B.; Raki, M.; Bergström, K.; von Rechenberg, B.; Miettinen, S. In vivo effects of bioactive glass s53p4 or beta tricalcium phosphate on osteogenic differentiation of human adipose stem cells after incubation with BMP-2. J. Stem Cell Res. Ther. 2012, 2, 1–11. [Google Scholar] [CrossRef]
- Alves, A.P.N.; Arango-Ospina, M.; Oliveira, R.L.M.S.; Ferreira, I.M.; Moraes, E.G.; Hartmann, M.; Oliveira, A.P.N.; Boccaccini, A.R.; Trichês, E.S. 3D-Printed β-TCP/S53P4 bioactive glass scaffolds coated with tea tree oil: Coating optimization, in vitro bioactivity and antibacterial properties. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Sabree, I.; Gough, J.E.; Derby, B. Mechanical properties of porous ceramic scaffolds: Influence of internal dimensions. Ceram. Int. 2015, 41, 8425–8432. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Marew, T.; Birhanu, G. Three Dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021, 18, 102–111. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, L.; de Paula, C.G.; Gouveia, R.F.; Motisuke, M.; de Sousa Trichês, E. Evaluation of the sintering temperature on the mechanical behavior of β-tricalcium phosphate/calcium silicate scaffolds obtained by gelcasting method. J. Mech. Behav. Biomed. Mater. 2019, 90, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.L.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Reid, J.W.; Pietak, A.; Sayer, M.; Dunfield, D.; Smith, T.J.N. Phase formation and evolution in the silicon substituted tricalcium phosphate/apatite system. Biomaterials 2005, 26, 2887–2897. [Google Scholar] [CrossRef]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef]
- Duncan, J.; Hayakawa, S.; Osaka, A.; MacDonald, J.F.; Hanna, J.V.; Skakle, J.M.S.; Gibson, I.R. Furthering the understanding of silicate-substitution in α-tricalcium phosphate: An X-ray diffraction, X-ray fluorescence and solid-state nuclear magnetic resonance study. Acta Biomater. 2014, 10, 1443–1450. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardi, M.; Bianco, A.; Ravaglioli, A.; Montanaro, L. Sol-Gel derived 45S5 Bioglass: Synthesis, microstructural evolution and thermal behaviour. J. Mater. Sci. Mater. Med. 2012, 23, 1849–1866. [Google Scholar] [CrossRef]
- Santos, S.C.; Barreto, L.S.; dos Santos, E.A. Nanocrystalline apatite formation on bioactive glass in a sol–gel synthesis. J. Non. Cryst. Solids 2016, 439, 30–37. [Google Scholar] [CrossRef]
- Hesaraki, S.; Safari, M.; Shokrgozar, M.A. Development of beta-tricalcium phosphate/sol-gel derived bioactive glass composites: Physical, mechanical, and in vitro biological evaluations. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Xin, R.; Leng, Y.; Chen, J.; Zhang, Q. A Comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials 2005, 26, 6477–6486. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium Phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Juhasz, J.A.; Best, S.M.; Bonfield, W.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Apatite-forming ability of glass-ceramic apatite-wollastonite—Polyethylene composites: Effect of filler content. J. Mater. Sci. Mater. Med. 2003, 14, 489–495. [Google Scholar] [CrossRef]
- Höland, W.; Beall, G.H. Glass-Ceramic Technology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Zheng, K.; Solodovnyk, A.; Li, W.; Goudouri, O.M.; Stähli, C.; Nazhat, S.N.; Boccaccini, A.R. Aging time and temperature effects on the structure and bioactivity of gel-derived 45S5 Glass-Ceramics. J. Am. Ceram. Soc. 2015, 98, 30–38. [Google Scholar] [CrossRef]
- Cowin, S.C. Bone poroelasticity. J. Biomech. 1999, 32, 217–238. [Google Scholar] [CrossRef]
- Hoover, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Silver doped resorbable tricalcium phosphate scaffolds for bone graft applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 763–769. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Kerr, L.; Bernstein, A.; Mayr, H.O.; Suedkamp, N.P.; Gadow, R.; Krieg, P.; Latorre, S.H.; Thomann, R.; Syrowatka, F.; et al. 3D powder printed bioglass and β-tricalcium phosphate bone scaffolds. Materials 2017, 11, 13. [Google Scholar] [CrossRef]
- Dutta Roy, T.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J. Biomed. Mater. Res. A 2003, 66, 283–291. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.S.; Zhou, W.S.; Xu, H.G.; Yang, M. Aspirin modified strontium-doped β-tricalcium phosphate can accelerate the healing of femoral metaphyseal defects in ovariectomized rats. Biomed. Pharmacother. 2020, 132, 110911. [Google Scholar] [CrossRef]
- Sarin, J.; Vuorenmaa, M.; Vallittu, P.K.; Grénman, R.; Boström, P.; Riihilä, P.; Nissinen, L.; Kähäri, V.M.; Pulkkinen, J. The viability and growth of hacat cells after exposure to bioactive glass s53p4-containing cell culture media. Otol. Neurotol. 2021, 42, e559–e567. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Chai, C.Y.; Loh, H.S. In vitro evaluation of osteoblast adhesion, proliferation and differentiation on chitosan-tio2 nanotubes scaffolds with Ca2+ ions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 144–152. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian, J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Chen, Y.P.; Chu, Y.L.; Tsuang, Y.H.; Wu, Y.; Kuo, C.Y.; Kuo, Y.J. Anti-inflammatory effects of adenine enhance osteogenesis in the osteoblast-like MG-63 Cells. Life 2020, 10, 116. [Google Scholar] [CrossRef]
- Prabakaran, S.; Rajan, M.; Lv, C.; Meng, G. Lanthanides-Substituted Hydroxyapatite/Aloe Vera composite coated titanium plate for bone tissue regeneration. Int. J. Nanomed. 2021, 16, 6535. [Google Scholar] [CrossRef] [PubMed]
- Xiaochen, L.; Wei, J.; Wei, S. Biological Effects of osteoblast-like cells on nanohydroxyapatite particles at a low concentration range. J. Nanomater. 2011, 2021, 104747. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pati, F.; Mandal, D.; Dhara, S.; Biswas, K. In vitro evaluation of osteoconductivity and cellular response of zirconia and alumina based ceramics. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3923–3930. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, M.; Zhang, M.; Ratner, B. Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003, 9, 337–345. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Atmaca, H. Biological response of osteoblastic and chondrogenic cells to graphene-containing pcl/bioactive glass bilayered scaffolds for osteochondral tissue engineering applications. Appl. Biochem. Biotechnol. 2018, 186, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Dong, Y.; Wang, S.; Gao, X.; Chen, X. A Novel nano-sized bioactive glass stimulates osteogenesis via the MAPK Pathway. RSC Adv. 2017, 7, 13760–13767. [Google Scholar] [CrossRef]
- Paramita, P.; Ramachandran, M.; Narashiman, S.; Nagarajan, S.; Sukumar, D.K.; Chung, T.W.; Ambigapathi, M. Sol-Gel based synthesis and biological properties of zinc integrated nano bioglass ceramics for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2021, 32, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, M.Y.; Ahn, H.; Kim, H.S.; Shin, H.I.; Jeong, D. Blocking of the ubiquitin-proteasome system prevents inflammation-induced bone loss by accelerating M-CSF receptor C-FMS degradation in osteoclast differentiation. Int. J. Mol. Sci. 2017, 18, 2054. [Google Scholar] [CrossRef] [PubMed]
- Mattson, A.M.; Begun, D.L.; Molstad, D.H.H.; Meyer, M.A.; Oursler, M.J.; Westendorf, J.J.; Bradley, E.W. Deficiency in the phosphatase PHLPP1 suppresses osteoclast-mediated bone resorption and enhances bone formation in mice. J. Biol. Chem. 2019, 294, 11772–11784. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Wolinetz, C.D.; Killian, G.J. Influence of Arginine-Glycine-Aspartic Acid (RGD), Integrins (AlphaV and Alpha5) and Osteopontin on bovine sperm-egg binding, and fertilization in vitro. Theriogenology 2007, 67, 468–474. [Google Scholar] [CrossRef]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W. Osteopontin: A leading candidate adhesion molecule for implantation in pigs and sheep. J. Anim. Sci. Biotechnol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell. Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52–54, 78–87. [Google Scholar] [CrossRef]
- Delany, A.M.; Amling, M.; Priemel, M.; Howe, C.; Baron, R.; Canalis, E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Investig. 2000, 105, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L.; Maeno, M.; Hawrylyshyn, B.; Yao, K.L.; Domenicucci, C.; Sodek, J. Differential effects of Transforming Growth Factor-Beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J. Cell Biol. 1988, 106, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Tarafder, S.; Vahabzadeh, S.; Bose, S. Effects of MgO, ZnO, SrO, and SiO2 in Tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 10–19. [Google Scholar] [CrossRef]
- Licini, C.; Vitale-Brovarone, C.; Mattioli-Belmonte, M. Collagen and non-collagenous proteins molecular crosstalk in the pathophysiology of osteoporosis. Cytokine Growth Factor Rev. 2019, 49, 59–69. [Google Scholar] [CrossRef]
- Guntur, A.R.; Rosen, C.J. The Skeleton: A multi-functional complex organ: New insights into osteoblasts and their role in bone formation: The central role of PI3Kinase. J. Endocrinol. 2011, 211, 123–130. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Blackwell, K.A.; Raisz, L.G.; Pilbeam, C.C. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol. Metab. 2010, 21, 294–301. [Google Scholar] [CrossRef]
- Brakebusch, C.; Hirsch, E.; Potocnik, A.; Fässler, R. Genetic analysis of beta1 integrin function: Confirmed, new and revised roles for a crucial family of cell adhesion molecules. J. Cell Sci. 1997, 110 Pt 23, 2895–2904. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Silva, J.C.; Hoff, C.M.; Cabral, J.M.S.; Linhardt, R.J.; da Silva, C.L.; Vashishth, D. Loss and rescue of osteocalcin and osteopontin modulate osteogenic and angiogenic features of mesenchymal stem/stromal cells. J. Cell. Physiol. 2020, 235, 7496–7515. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.; van Vugt, T.; Thijssen, E.; Arts, J.J. Cost-effectiveness study of one-stage treatment of chronic osteomyelitis with bioactive glass S53P4. Materials 2019, 12, 3209. [Google Scholar] [CrossRef]
- Gonzalez Moreno, M.; Butini, M.E.; Maiolo, E.M.; Sessa, L.; Trampuz, A. Antimicrobial activity of bioactive glass S53P4 against representative microorganisms causing osteomyelitis—Real-time assessment by isothermal microcalorimetry. Colloids Surf. B Biointerfaces 2020, 189, 110853. [Google Scholar] [CrossRef]
- Grønseth, T.; Vestby, L.K.; Nesse, L.L.; von Unge, M.; Silvola, J.T. Bioactive Glass S53P4 eradicates Staphylococcus Aureus in biofilm/planktonic states in vitro. Ups. J. Med. Sci. 2020, 125, 217–225. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Arweiler-Harbeck, D.; Arnolds, J.; Hussain, T.; Hansen, S.; Bertram, R.; Buer, J.; Lang, S.; Steinmann, J.; Höing, B. Imaging studies of bacterial biofilms on cochlear implants-bioactive glass (BAG) inhibits mature biofilm. PLoS ONE 2020, 15, e0229198. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.M.; Rekola, J.; Hirvonen, J.; Aho, A.J. Comparison of the osteoconductive properties of three particulate bone fillers in a rabbit model: Allograft, calcium carbonate (Biocoral®) and S53P4 Bioactive Glass. Acta Odontol. Scand. 2013, 71, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Bellato, C.P.; de Oliveira, D.L.; Kasaya, M.V.S.; Moreira, D.; Cini, M.A.; Saraiva, P.P.; Gulinelli, J.L.; Santos, P.L. Effect of S53P4 bioactive glass and low-level laser therapy on calvarial bone repair in rats submitted to zoledronic acid therapy. Acta Cir. Bras. 2021, 36, e360603. [Google Scholar] [CrossRef]
- Eriksson, E.; Björkenheim, R.; Strömberg, G.; Ainola, M.; Uppstu, P.; Aalto-Setälä, L.; Leino, V.M.; Hupa, L.; Pajarinen, J.; Lindfors, N.C. S53P4 bioactive glass scaffolds induce BMP expression and integrative bone formation in a critical-sized diaphysis defect treated with a single-staged induced membrane technique. Acta Biomater. 2021, 126, 463–476. [Google Scholar] [CrossRef]









| Gene | PubMed Reference | Forward Primer | Reverse Primer | Product Length (pb) |
|---|---|---|---|---|
| Col-1 | NM_000088.3 | ACAGCCGCTTCACCTACAGC | GTTTTGTATTCAATCACTGTCTTGC | 85 |
| Tgf-β1 | NM_000660.4 | TTTGATGTCACCGGAGTTGTG | GCGAAAGCCCTCAATTTCC | 63 |
| Itg β1 | NM_002211.3 | TTCTTCCTGGACTATTGAAAT | AGAAACTCTCATCATGCTCATT | 100 |
| M-csf | NM_172211.3 | GAGCTGCTTCACCAAGGATTAT | TCTTGACCTTCTCCAGCAACTG | 92 |
| Osn | NM_003118.3 | ACTGGCTCAAGAACGTCCTGGT | TCATGGATCTTCTTCACCCGC | 97 |
| Bglap | NM_001199662.1 | AGCAGAGCGACACCCTAGAC | GGCAGCGAGGTAGTGAAGAG | 194 |
| Osp | NM_001251830.1 | AGACACATATGATGGCCGAGG | GGCCTTGTATGCACCATTCAA | 154 |
| PgE2 | NM_004878.4 | GAAGAAGGCCTTTGCCAA | GGAAGACCAGGAAGTGC | 200 |
| Runx2 | NM_001015051 | GAACTGGGCCCTTTTTCAGA | CACTCTGGCTTTGGGAAGAG | 208 |
| β-actin | NM_001101.3 | AAACTGGAACGGTGAAGGTG | GTGGACTTGGGAGAGGACTG | 206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, S.S.; Lima, B.S.d.S.; Avelino, S.O.M.; Spirandeli, B.R.; Campos, T.M.B.; Thim, G.P.; Trichês, E.d.S.; Prado, R.F.d.; Vasconcellos, L.M.R.d. β-TCP/S53P4 Scaffolds Obtained by Gel Casting: Synthesis, Properties, and Biomedical Applications. Bioengineering 2023, 10, 597. https://doi.org/10.3390/bioengineering10050597
Amaral SS, Lima BSdS, Avelino SOM, Spirandeli BR, Campos TMB, Thim GP, Trichês EdS, Prado RFd, Vasconcellos LMRd. β-TCP/S53P4 Scaffolds Obtained by Gel Casting: Synthesis, Properties, and Biomedical Applications. Bioengineering. 2023; 10(5):597. https://doi.org/10.3390/bioengineering10050597
Chicago/Turabian StyleAmaral, Suelen Simões, Beatriz Samara de Sousa Lima, Sarah Oliveira Marco Avelino, Bruno Roberto Spirandeli, Tiago Moreira Bastos Campos, Gilmar Patrocínio Thim, Eliandra de Sousa Trichês, Renata Falchete do Prado, and Luana Marotta Reis de Vasconcellos. 2023. "β-TCP/S53P4 Scaffolds Obtained by Gel Casting: Synthesis, Properties, and Biomedical Applications" Bioengineering 10, no. 5: 597. https://doi.org/10.3390/bioengineering10050597
APA StyleAmaral, S. S., Lima, B. S. d. S., Avelino, S. O. M., Spirandeli, B. R., Campos, T. M. B., Thim, G. P., Trichês, E. d. S., Prado, R. F. d., & Vasconcellos, L. M. R. d. (2023). β-TCP/S53P4 Scaffolds Obtained by Gel Casting: Synthesis, Properties, and Biomedical Applications. Bioengineering, 10(5), 597. https://doi.org/10.3390/bioengineering10050597