Imaging Phenotypes and Evolution of Hepatic Langerhans Cell Histiocytosis on CT/MRI: A Retrospective Study of Clinical Cases and Literature Review
Abstract
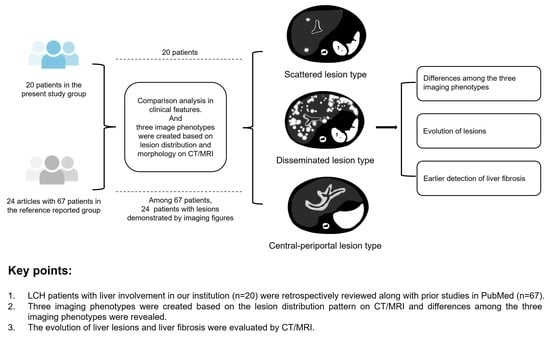
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CT and MRI Examinations
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients and Clinical Characteristics
3.2. Image Phenotypes Based on Lesion Distribution
3.3. Detailed Imaging Findings and Evolution of Lesions
3.3.1. Parenchymal Lesions
- Evolution of parenchymal lesions in eight patients with multiple MRI scans
3.3.2. Periportal Abnormalities
- Evolution of periportal lesions in eight patients with multiple MRI scans
3.3.3. Hepatic Fibrosis and Other Imaging Findings
4. Discussion
4.1. Differences among the Three Imaging Phenotypes: Patient Age, Clinical Feature, and Fibrosis
- Central periportal lesion phenotype
- Disseminated lesion phenotype
- Scattered lesion phenotype
4.2. Evolution of Lesions
4.3. Detailed Additions to Imaging Findings
4.4. Earlier Detection of Liver Fibrosis
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, G.; Young, J.R.; Koster, M.J.; Tobin, W.O.; Vassallo, R.; Ryu, J.H.; Davidge-Pitts, C.J.; Hurtado, M.D.; Ravindran, A.; Sartori Valinotti, J.C.; et al. The Mayo Clinic Histiocytosis Working Group Consensus Statement for the Diagnosis and Evaluation of Adult Patients With Histiocytic Neoplasms: Erdheim-Chester Disease, Langerhans Cell Histiocytosis, and Rosai-Dorfman Disease. Mayo Clin. Proc. 2019, 94, 2054–2071. [Google Scholar] [CrossRef]
- McClain, K.L.; Bigenwald, C.; Collin, M.; Haroche, J.; Marsh, R.A.; Merad, M.; Picarsic, J.; Ribeiro, K.B.; Allen, C.E. Histiocytic disorders. Nat. Rev. Dis. Prim. 2021, 7, 73. [Google Scholar] [CrossRef]
- Emile, J.F.; Abla, O.; Fraitag, S.; Horne, A.; Haroche, J.; Donadieu, J.; Requena-Caballero, L.; Jordan, M.B.; Abdel-Wahab, O.; Allen, C.E.; et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 2016, 127, 2672–2681. [Google Scholar] [CrossRef]
- Abdallah, M.; Généreau, T.; Donadieu, J.; Emile, J.F.; Chazouillères, O.; Gaujoux-Viala, C.; Cabane, J. Langerhans’ cell histiocytosis of the liver in adults. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.R.; Fraum, T.J.; Ballard, D.H.; Narra, V.R.; Shetty, A.S. Imaging Biomarkers of Hepatic Fibrosis: Reliability and Accuracy of Hepatic Periportal Space Widening and Other Morphologic Features on MRI. AJR Am. J. Roentgenol. 2021, 216, 1229–1239. [Google Scholar] [CrossRef]
- Girschikofsky, M.; Arico, M.; Castillo, D.; Chu, A.; Doberauer, C.; Fichter, J.; Haroche, J.; Kaltsas, G.A.; Makras, P.; Marzano, A.V.; et al. Management of adult patients with Langerhans cell histiocytosis: Recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J. Rare Dis. 2013, 8, 72. [Google Scholar] [CrossRef]
- Haupt, R.; Minkov, M.; Astigarraga, I.; Schäfer, E.; Nanduri, V.; Jubran, R.; Egeler, R.M.; Janka, G.; Micic, D.; Rodriguez-Galindo, C.; et al. Langerhans cell histiocytosis (LCH): Guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr. Blood Cancer 2013, 60, 175–184. [Google Scholar] [CrossRef]
- Weitzman, S.; Egeler, R.M. Langerhans cell histiocytosis: Update for the pediatrician. Curr. Opin. Pediatr. 2008, 20, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Debnath, J.; Thulkar, S.; Seth, T.; Sinha, A. Imaging findings in hepatic Langerhans’ cell histiocytosis. Indian J. Pediatr. 2006, 73, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Eich, G.; Geoffray, A.; Hanquinet, S.; Waibel, P.; Wolf, R.; Letovanec, I.; Alamo-Maestre, L.; Gudinchet, F. Extraosseous langerhans cell histiocytosis in children. Radiographic 2008, 28, 707–726. [Google Scholar] [CrossRef]
- Arakawa, A.; Matsukawa, T.; Yamashita, Y.; Yoshimatsu, S.; Ohtsuka, N.; Miyazaki, T.; Yamamoto, H.; Harada, M.; Ishimaru, Y.; Takahashi, M. Periportal fibrosis in Langerhans’ cell histiocytosis mimicking multiple liver tumors: US, CT, and MR findings. J. Comput. Assist. Tomogr. 1994, 18, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Mampaey, S.; Warson, F.; Van Hedent, E.; De Schepper, A.M. Imaging findings in Langerhans’ cell histiocytosis of the liver and the spleen in an adult. Eur. Radiol. 1999, 9, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Hizawa, N.; Betsuyaku, T.; Yasuo, M.; Yamamoto, H.; Koizumi, T.; Nishimura, M. Adult Langerhans cell histiocytosis with independently relapsing lung and liver lesions that was successfully treated with etoposide. Intern. Med. 2007, 46, 1231–1235. [Google Scholar] [CrossRef]
- Savva-Bordalo, J.; Freitas-Silva, M. Langerhans cell histiocytosis involving the liver of a male smoker: A case report. J. Med. Case Rep. 2008, 2, 376. [Google Scholar] [CrossRef]
- Hu, X.; Dong, A.; Lv, S.; Wang, Q.; Zhan, X.; Song, X.; Wang, J. F-18 FDG PET/CT imaging of solitary liver Langerhans cell histiocytosis: Preliminary findings. Ann. Nucl. Med. 2012, 26, 436–439. [Google Scholar] [CrossRef]
- Yuasa, M.; Fujiwara, S.; Oh, I.; Yamaguchi, T.; Fukushima, N.; Morimoto, A.; Ozawa, K. Rapidly progressing fatal adult multi-organ Langerhans cell histiocytosis complicated with fatty liver disease. J. Clin. Exp. Hematop. JCEH 2012, 52, 121–126. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Y.; Chen, X.; Gong, G. Langerhans cell histiocytosis misdiagnosed as liver cancer and pituitary tumor in an adult: A case report and brief review of the literature. Oncol. Lett. 2014, 7, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Araujo, B.; Costa, F.; Lopes, J.; Castro, R. Adult langerhans cell histiocytosis with hepatic and pulmonary involvement. Case Rep. Radiol. 2015, 2015, 536328. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Weng, S.Q.; Dong, L.; Shen, X.Z. Eosinophilic pseudotumor of the liver: Report of six cases and review of literature. J. Dig. Dis. 2015, 16, 159–163. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Z.; Chen, M.; Ju, W.; Wang, D.; Ji, F.; Ren, Q.; Guo, Z.; He, X. Severe sclerosing cholangitis after Langerhans cell histiocytosis treated by liver transplantation: An adult case report. Medicine 2017, 96, e5994. [Google Scholar] [CrossRef]
- Ouizeman, D.J.; Patouraux, S.; Lacour, J.P.; Anty, R. A decompensated cryptogenic cirrhosis? No, a late liver histiocytosis! Dig. Liver Dis. 2018, 50, 1369. [Google Scholar] [CrossRef]
- Wang, B.B.; Ye, J.R.; Li, Y.L.; Jin, Y.; Chen, Z.W.; Li, J.M.; Li, Y.P. Multisystem involvement Langerhans cell histiocytosis in an adult: A case report. World J. Clin. Cases 2020, 8, 4966–4974. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.J.; Shahi, R.R.; Maharjan, S.; Sharma, S.; Sudhir Suman, K.C. Muscle Infiltrative Adult Multisystem Langerhans Cell Histiocytosis Detected on Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography—A Rare Case. Indian J. Nucl. Med. 2020, 35, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy Rajavelu, T.; Abimannane, A.; Chinnaiah Govindhareddy, D.K.; Kayal, S.; Kar, R. Langerhans’ Cell Histiocytosis Masquerading as Caroli’s Disease. J. Pediatr. Hematol./Oncol. 2020, 42, e620–e622. [Google Scholar] [CrossRef]
- Kapoor, R.; Loizides, A.M.; Sachdeva, S.; Paul, P. Disseminated langerhans cell histiocytosis presenting as cholestatic jaundice. J. Clin. Diagn. Res. JCDR 2015, 9, Sd03–Sd05. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Qiao, Z.; Xia, C.; Gong, Y.; Yang, H.; Li, G.; Pa, M. Hepatic involvement of Langerhans cell histiocytosis in children--imaging findings of computed tomography, magnetic resonance imaging and magnetic resonance cholangiopancreatography. Pediatr. Radiol. 2014, 44, 713–718. [Google Scholar] [CrossRef]
- Wong, A.; Ortiz-Neira, C.L.; Reslan, W.A.; Sharon, R.; Pinto-Rojas, A.; Kaura, D.; Anderson, R. Liver involvement in Langerhans cell histiocytosis. Pediatr. Radiol. 2006, 36, 1105–1107. [Google Scholar] [CrossRef]
- Kim, M.; Lyu, C.; Jin, Y.; Yoo, H. Langerhans’ cell histiocytosis as a cause of periportal abnormal signal intensity on MRI. Abdom. Imaging 1999, 24, 373–377. [Google Scholar] [CrossRef]
- Chan, Y.L.; Li, C.K.; Lee, C.Y. Sonographic appearance of hepatic Langerhans cell histiocytosis. Clin. Radiol. 1997, 52, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P. Liver biopsy. Gastroenterol. Clin. Biol. 2008, 32, 4–7. [Google Scholar] [CrossRef]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 11033–11053. [Google Scholar] [CrossRef]
- Bedossa, P.; Dargère, D.; Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Galea, N.; Cantisani, V.; Taouli, B. Liver lesion detection and characterization: Role of diffusion-weighted imaging. J. Magn. Reson. Imaging 2013, 37, 1260–1276. [Google Scholar] [CrossRef]
- Taouli, B.; Koh, D.M. Diffusion-weighted MR imaging of the liver. Radiology 2010, 254, 47–66. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, J.; Gao, R.; Huang, Z.; Wu, M.; Song, B. Liver fibrosis staging with diffusion-weighted imaging: A systematic review and meta-analysis. Abdom. Radiol. 2017, 42, 490–501. [Google Scholar] [CrossRef]
- Feier, D.; Balassy, C.; Bastati, N.; Fragner, R.; Wrba, F.; Ba-Ssalamah, A. The diagnostic efficacy of quantitative liver MR imaging with diffusion-weighted, SWI, and hepato-specific contrast-enhanced sequences in staging liver fibrosis--a multiparametric approach. Eur. Radiol. 2016, 26, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Venkatesh, S.H.; Eaton, J.E.; Bolan, C.W.; Osman, K.T.; Maselli, D.B.; Menias, C.O.; Venkatesh, S.K. Magnetic resonance imaging features in 283 patients with primary biliary cholangitis. Eur. Radiol. 2020, 30, 5139–5148. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Sukonrut, K.; Korpraphong, P.; Pongpaibul, A.; Saiviroonporn, P. Diffusion-weighted magnetic resonance imaging for the assessment of liver fibrosis in chronic viral hepatitis. PLoS ONE 2021, 16, e0248024. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Energy. European Guidelines on Diagnostic Reference Levels for Paediatric Imaging; Radiation Protection No. 185; EU Publication Office: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Faria, S.C.; Ganesan, K.; Mwangi, I.; Shiehmorteza, M.; Viamonte, B.; Mazhar, S.; Peterson, M.; Kono, Y.; Santillan, C.; Casola, G.; et al. MR imaging of liver fibrosis: Current state of the art. Radiographic 2009, 29, 1615–1635. [Google Scholar] [CrossRef]
- Chen, X.; Qin, L.; Pan, D.; Huang, Y.; Yan, L.; Wang, G.; Liu, Y.; Liang, C.; Liu, Z. Liver diffusion-weighted MR imaging: Reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology 2014, 271, 113–125. [Google Scholar] [CrossRef]
- Gupta, A.K. Imaging findings in Langerhans cell histiocytosis involving lung and liver. Indian J. Pediatr. 2009, 76, 560–561. [Google Scholar] [CrossRef]
- Buza, N.; Lagarde, D.C.; Dash, S.; Haque, S. Langerhans cell histiocytosis: Report of a single organ involvement in a child. J. Cell. Mol. Med. 2004, 8, 397–401. [Google Scholar] [CrossRef]
- Caruso, S.; Miraglia, R.; Maruzzelli, L.; Luca, A.; Gridelli, B. Biliary wall calcification in Langerhans cell histiocytosis: Report of two cases. Pediatr. Radiol. 2008, 38, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ells, P.; Arslan, M.E.; Robstad, K.A.; Lee, H. Hepatic Langerhans Cell Histiocytosis (LCH) Presenting as a Harbinger of Multisystem LCH. Cureus 2020, 12, e8591. [Google Scholar] [CrossRef]
- Heyn, R.M.; Hamoudi, A.; Newton, W.A., Jr. Pretreatment liver biopsy in 20 children with histiocytosis X: A clinicopathologic correlation. Med. Pediatr. Oncol. 1990, 18, 110–118. [Google Scholar] [CrossRef]
- Radin, D.R. Langerhans cell histiocytosis of the liver: Imaging findings. AJR. Am. J. Roentgenol. 1992, 159, 63–64. [Google Scholar] [CrossRef]
- König, C.W.; Pfannenberg, C.; Trübenbach, J.; Remy, C.; Böhmer, G.M.; Ruck, P.; Claussen, C.D. MR cholangiography in the diagnosis of sclerosing cholangitis in Langerhans’ cell histiocytosis. Eur. Radiol. 2001, 11, 2516–2520. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, N.; Erden, A.; Erden, I. Primary biliary cirrhosis: Evaluation with T2-weighted MR imaging and MR cholangiopancreatography. Eur. J. Radiol. 2009, 69, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.S.; Donohoe, A.; Ford, K.L., 3rd; Glastad, K.; Watkins, D.; Molmenti, E. Primary biliary cirrhosis: MR imaging findings and description of MR imaging periportal halo sign. AJR. Am. J. Roentgenol. 2001, 176, 885–889. [Google Scholar] [CrossRef]
- Roberts, P.; Trout, A.T.; Dillman, J.R. Nodular macroregenerative tissue as a pattern of regeneration in cholangiopathic disorders. Pediatr. Radiol. 2018, 48, 932–940. [Google Scholar] [CrossRef]
- Kovač, J.D.; Ješić, R.; Stanisavljević, D.; Kovač, B.; Banko, B.; Seferović, P.; Maksimović, R. Integrative role of MRI in the evaluation of primary biliary cirrhosis. Eur. Radiol. 2012, 22, 688–694. [Google Scholar] [CrossRef]
- Ding, Y.; Rao, S.; Yang, L.; Chen, C.; Zeng, M. Comparison of the effect of region-of-interest methods using gadoxetic acid-enhanced MR imaging with diffusion-weighted imaging on staging hepatic fibrosis. Radiol. Med. 2016, 121, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.X.; Huang, H.; Yuan, J.; Zhao, F.; Chen, Z.Y.; Zhang, Q.; Ahuja, A.T.; Zhou, B.P.; Wáng, Y.X. Decreases in molecular diffusion, perfusion fraction and perfusion-related diffusion in fibrotic livers: A prospective clinical intravoxel incoherent motion MR imaging study. PLoS ONE 2014, 9, e113846. [Google Scholar] [CrossRef]
- Patel, J.; Sigmund, E.E.; Rusinek, H.; Oei, M.; Babb, J.S.; Taouli, B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: Preliminary experience. J. Magn. Reson. Imaging JMRI 2010, 31, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Baek, J.H.; Shin, C.I.; Kiefer, B.; Han, J.K.; Choi, B.I. Evaluation of hepatic fibrosis using intravoxel incoherent motion in diffusion-weighted liver MRI. J. Comput. Assist. Tomogr. 2014, 38, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Facciorusso, A.; Loomba, R.; Falck-Ytter, Y.T. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2018, 16, 27–38.e24. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liang, Y.; Liu, M. The value of MRI in the diagnosis of primary biliary cirrhosis and assessment of liver fibrosis. PLoS ONE 2015, 10, e0120110. [Google Scholar] [CrossRef]




| Characteristics | Combined a | p-Value 1 b | Reference Reported c | Present Study | Scattered Lesion Type in Present Study | Disseminated Lesion Type in Present Study | Central Periportal Lesion Type in Present Study | p-Value 2 d |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 87 | 67 | 20 | 6 | 8 | 6 | ||
| Sex, n (%) | 0.851 | 1.000 | ||||||
| Male | 55 (63.2) | 42 (62.7) | 13 (65) | 4 (66.7) | 5 (62.5) | 4 (66.7) | ||
| Female | 32 (36.8) | 25 (37.3) | 7 (35) | 2 (33.3) | 3 (37.5) | 2 (33.3) | ||
| Age at diagnosis, n (%) | 0.620 | <0.001 * | ||||||
| ≥15 Y | 52 (59.8) | 41 (61.2) | 11 (55) | 6 (100) | 5 (62.5) | 0 | ||
| <15 Y | 35 (40.2) | 26 (38.8) | 9 (45) | 0 | 3 (37.5) | 6 (100) | ||
| Mean ± SD, (Years) | NA | NA | 19.5 ± 16.5 | 36.8 ± 8.4 | 19.7 ± 12.9 | 1.8 ± 0.4 | ||
| Range, (Years) | NA | NA | 1.1~53 | 29~53 | 1.1~39 | 1.2~2.5 | ||
| Liver involvement, n(%) | / | / | ||||||
| At baseline | NA | NA | 18 (90) | 4 (66.7) | 8 (100) | 6 (100) | ||
| NA | NA | NA | 2 (10) | 2 (33.3) | 0 | 0 | ||
| Hepatomegaly, n (%) | 42 (62.7) | 0.798 | 29 (61.7) e | 13 (65.0) | 1 (16.7) | 7 (87.5) | 5 (83.3) | 0.019 * |
| Liver biochemical abnormalities, n (%) | 54 (74.0) | 0.471 | 38 (71.7) f | 16 (80.0) | 2 (33.3) | 8 (100.0) | 6 (100.0) | 0.006 * |
| Stratification, n (%) | 1.000 | 1.000 | ||||||
| MS-LCH | 78 (96.3) | 59 (96.7) g | 19 (95) | 6 (100) | 7 (87.5) | 6 (100) | ||
| SS-LCH | 3 (3.7) | 2 (3.3) | 1 (5) | 0 | 1 (12.5) | 0 | ||
| Other organ, n (%) | / | 0.004 * | ||||||
| Bones | NA | NA | 10 (50) | 5 (83.3) | 2 (25) | 3 (50) | ||
| Lung | NA | NA | 8 (40) | 2 (33.3) | 5 (62.5) | 1 (16.7) | ||
| Skin | NA | NA | 8 (40) | 0 | 3 (37.5) | 5 (83.3) | ||
| Lymph node | NA | NA | 4 (20) | 1 (16.7) | 0 | 3 (50) | ||
| pituitary | NA | NA | 6 (30) | 1 (16.7) | 5 (62.5) | 0 | ||
| Thymus | NA | NA | 1 (5) | 0 | 1 (12.5) | 0 | ||
| Thyroid | NA | NA | 3 (15) | 1 (16.7) | 2 (25) | 0 | ||
| bone marrow | NA | NA | 2 (10) | 0 | 1 (12.5) | 1 (16.7) | ||
| Mucosa | NA | NA | 3 (15) | 0 | 0 | 3 (50) | ||
| Nervous system | NA | NA | 2 (10) | 1 (16.7) | 1 (12.5) | 0 | ||
| Ear | NA | NA | 2 (10) | 0 | 0 | 2 (33.3) | ||
| Genitalia | NA | NA | 1 (5) | 0 | 1 (12.5) | 0 | ||
| Spleen | NA | NA | 3 (15) | 0 | 1 (12.5) | 2 (33.3) |
| Liver Biochemical Results | Scattered Lesion Phenotype | Disseminated Lesion Phenotype | Central Periportal Lesion Phenotype | p-Value |
|---|---|---|---|---|
| Number of patients | 6 | 8 | 6 | |
| γGT, AP | <0.001 * | |||
| Normal | 4 | 0 | 0 | |
| γGT increased ≤2 times normal/AP increased ≤1.5 times normal | 2 | 2 | 0 | |
| γGT increased >2 times normal/AP increased >1.5 times normal | 0 | 6 | 6 | |
| ALT, AST | 0.025 * | |||
| Normal | 5 | 2 | 0 | |
| ALT/AST increased ≤3 times normal | 1 | 4 | 1 | |
| 3 times normal <ALT/AST increased ≤5 times normal | 0 | 1 | 2 | |
| ALT/AST increased >5 times normal | 0 | 1 | 3 | |
| Albumin | 0.066 | |||
| Normal | 6 | 6 | 2 | |
| Hypoalbuminemia >30 g/dl | 0 | 2 | 4 | |
| Hypoalbuminemia <30 g/dl | 0 | 0 | 0 | |
| Bilirubin | 0.01 * | |||
| Normal | 6 | 6 | 1 | |
| Hyperbilirubinemia ≤3 times normal | 0 | 1 | 4 | |
| 3 times normal < hyperbilirubinemia ≤5 times normal | 0 | 1 | 0 | |
| Hyperbilirubinemia >5 times normal | 0 | 0 | 1 |
| Accompanied Imaging Signs | All Patients | Scattered Lesion Type | Disseminated Lesion Type | Central Periportal Lesion Type | p Value b |
|---|---|---|---|---|---|
| Number of patients | 20 | 6 | 8 | 6 | |
| T2/DWI hypointense, n (%) | 0.019 * | ||||
| Periportal halo sign | 2 (13) | 0 | 0 | 2 (33.3) | |
| Giant, central nodular fibrosis | 1 (6.7) | 0 | 0 | 1 (16.7) | |
| Patchy fibrosis | 6 (40) | 0 | 3 (60) | 3 (50) | |
| NA a | 5 (25) | 2 (33.3) | 3 (37.5) | 0 | |
| Hepatic surface, n (%) | 0.035 * | ||||
| Smooth | 17 (85) | 6 (100) | 8 (100) | 3 (50) | |
| Nodular | 3 (15) | 0 | 0 | 3 (50) | |
| Hepatomegaly, n (%) | 13 (65) | 1 (16.7) | 7 (87.5) | 5 (83.3) | 0.019 * |
| Splenomegaly, n (%) | 12 (60) | 1 (16.7) | 6 (75) | 5 (83.3) | 0.053 |
| Enlarged lymph Nodes, n (%) | 11 (55) | 0 | 7 (87.5) | 4 (66.7) | 0.005 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Li, Y.; Xiong, Z.; Jiang, Y.; Hu, X.; Hu, D.; Li, Z.; Shen, Y. Imaging Phenotypes and Evolution of Hepatic Langerhans Cell Histiocytosis on CT/MRI: A Retrospective Study of Clinical Cases and Literature Review. Bioengineering 2023, 10, 598. https://doi.org/10.3390/bioengineering10050598
Hao L, Li Y, Xiong Z, Jiang Y, Hu X, Hu D, Li Z, Shen Y. Imaging Phenotypes and Evolution of Hepatic Langerhans Cell Histiocytosis on CT/MRI: A Retrospective Study of Clinical Cases and Literature Review. Bioengineering. 2023; 10(5):598. https://doi.org/10.3390/bioengineering10050598
Chicago/Turabian StyleHao, Luwen, Yuanqiu Li, Ziman Xiong, Yuchen Jiang, Xuemei Hu, Daoyu Hu, Zhen Li, and Yaqi Shen. 2023. "Imaging Phenotypes and Evolution of Hepatic Langerhans Cell Histiocytosis on CT/MRI: A Retrospective Study of Clinical Cases and Literature Review" Bioengineering 10, no. 5: 598. https://doi.org/10.3390/bioengineering10050598
APA StyleHao, L., Li, Y., Xiong, Z., Jiang, Y., Hu, X., Hu, D., Li, Z., & Shen, Y. (2023). Imaging Phenotypes and Evolution of Hepatic Langerhans Cell Histiocytosis on CT/MRI: A Retrospective Study of Clinical Cases and Literature Review. Bioengineering, 10(5), 598. https://doi.org/10.3390/bioengineering10050598