Advances and Challenges in Wearable Glaucoma Diagnostics and Therapeutics
Abstract
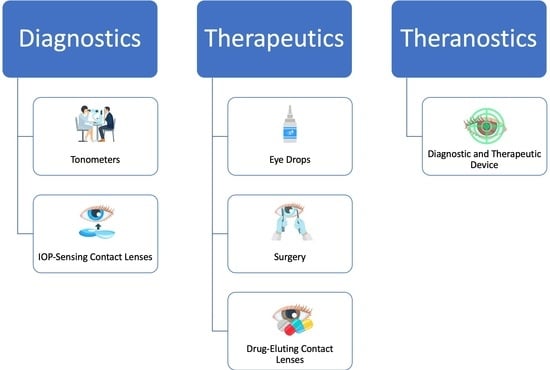
:1. Introduction
2. Diagnostics of Glaucoma
2.1. Current Methods for Measuring IOP
2.1.1. Goldmann Applanation Tonometer
2.1.2. Handheld Tonometers
2.1.3. Sensimed Triggerfish and GlakoLens
2.2. Recent Advances in Wearable Diagnostics
3. Therapeutics
3.1. Current Methods for Lowering IOP
3.1.1. Eye Drops
3.1.2. Intracameral Implants
3.1.3. Lasers and Surgery
3.2. Recent Advances in Wearable Therapeutics
4. Emerging Theranostic Platform
4.1. Description of Theranostics
4.2. Clinical Uses of Theranostics
4.3. Emerging Technologies
5. Current Challenges and Clinical Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Blindness and Visual Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 26 April 2023).
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668. [Google Scholar]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Early Manifest Glaucoma Trial Group. Early Manifest Glaucoma Trial: Design and baseline data. Ophthalmology 1999, 106, 2144–2153. [Google Scholar] [CrossRef]
- Grippo, T.M.; Liu, J.H.K.; Zebardast, N.; Arnold, T.B.; Moore, G.H.; Weinreb, R.N. Twenty-four–hour pattern of intraocular pressure in untreated patients with ocular hypertension. Investig. Opthalmol. Vis. Sci. 2013, 54, 512–517. [Google Scholar] [CrossRef]
- Matlach, J.; Bender, S.; König, J.; Binder, H.; Pfeiffer, N.; Hoffmann, E.M. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin. Ophthalmol. 2019, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Caprioli, J. Intraocular pressure fluctuation: Is it important? J. Ophthalmic Vis. Res. 2018, 13, 170. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. A review on flexible wearables-Recent developments in non-invasive continuous health monitoring. Sens. Actuators A Phys. 2024, 366, 114993. [Google Scholar] [CrossRef]
- Wu, K.Y.; Mina, M.; Carbonneau, M.; Marchand, M.; Tran, S.D. Advancements in Wearable and Implantable Intraocular Pressure Biosensors for Ophthalmology: A Comprehensive Review. Micromachines 2023, 14, 1915. [Google Scholar] [CrossRef]
- Zeppieri, M.; Gurnani, B. Applanation Tonometry. Available online: https://www.ncbi.nlm.nih.gov/books/NBK582132/ (accessed on 13 July 2023).
- Tonnu, P.-A.; Ho, T.; Sharma, K.; White, E.; Bunce, C.; Garway-Heath, D. A comparison of four methods of tonometry: Method agreement and interobserver variability. Br. J. Ophthalmol. 2005, 89, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Garg, A.; Vickerstaff, V.; Hunter, R.; Ambler, G.; Bunce, C.; Wormald, R.; Nathwani, N.; et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre randomised controlled trial. Lancet 2019, 393, 1505–1516. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.-L.; Bian, A.-L.; Liu, X.-L.; Jin, Y.-M. Effects of central corneal thickness and corneal curvature on measurement of intraocular pressure with Goldmann applanation tonometer and non-contact tonometer. Zhonghua Yan Ke Za Zhi Chin. J. Ophthalmol. 2009, 45, 713–718. [Google Scholar]
- Stodtmeister, R. Applanation tonometry and correction according to corneal thickness. Acta Ophthalmol. Scand. 1998, 76, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.E.B.; Pye, D.C.M.; Hali, A.B.; Lin, C.B.; Kam, P.B.; Ngyuen, T.B. The effect of contact lens induced corneal edema on Goldmann applanation tonometry measurements. J. Glaucoma 2007, 16, 153–158. [Google Scholar] [CrossRef]
- Stamper, R.L. A history of intraocular pressure and its measurement. Optom. Vis. Sci. 2011, 88, E16–E28. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Pardo, P.; Mendez-Hernández, C.; Valls-Ferran, I.; Puertas-Bordallo, D. Icare-Pro rebound tonometer versus hand-held applanation tonometer for pediatric screening. J. Pediatr. Ophthalmol. Strabismus 2018, 55, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Agrawal, A.; Pal, V.K.; Pratap, V.B. Rebound tonometer: Ideal tonometer for measurement of accurate intraocular pressure. J. Glaucoma 2014, 23, 633–637. [Google Scholar] [CrossRef]
- Pakrou, N.; Gray, T.; Mills, R.; Landers, J.; Craig, J. Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J. Glaucoma 2008, 17, 43–47. [Google Scholar] [CrossRef]
- Ting, S.L.; Lim, L.T.; Ooi, C.Y.; Rahman, M.M. Comparison of icare rebound tonometer and Perkins applanation tonometer in community eye screening. Asia-Pacific J. Ophthalmol. 2019, 8, 229–232. [Google Scholar] [CrossRef]
- Galgauskas, S.; Strupaite, R.; Strelkauskaite, E.; Asoklis, R. Comparison of intraocular pressure measurements with different contact tonometers in young healthy persons. Int. J. Ophthalmol. 2016, 9, 76. [Google Scholar] [CrossRef]
- Poostchi, A.; Mitchell, R.; Nicholas, S.; Purdie, G.; Wells, A. The iCare rebound tonometer: Comparisons with Goldmann tonometry, and influence of central corneal thickness. Clin. Exp. Ophthalmol. 2009, 37, 687–691. [Google Scholar] [CrossRef]
- Chui, W.-S.; Lam, A.; Chen, D.; Chiu, R. The influence of corneal properties on rebound tonometry. Ophthalmology 2008, 115, 80–84. [Google Scholar] [CrossRef]
- Fernandes, P.; Díaz-Rey, J.A.; Queirós, A.; Gonzalez-Meijome, J.M.; Jorge, J. Comparison of the ICare® rebound tonometer with the Goldmann tonometer in a normal population. Ophthalmic Physiol. Opt. 2005, 25, 436–440. [Google Scholar] [CrossRef]
- Cvenkel, B.; Velkovska, M.A.; Jordanova, V.D. Self-measurement with Icare HOME tonometer, patients’ feasibility and acceptability. Eur. J. Ophthalmol. 2020, 30, 258–263. [Google Scholar] [CrossRef]
- Leonardi, M.; Leuenberger, P.; Bertrand, D.; Bertsch, A.; Renaud, P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Investig. Opthalmology Vis. Sci. 2004, 45, 3113–3117. [Google Scholar] [CrossRef]
- Tong, J.B.; Huang, J.P.; Kalloniatis, M.P.; Coroneo, M.M.; Zangerl, B.P. Clinical trial: Diurnal IOP fluctuations in glaucoma using Latanoprost and Timolol with self-tonometry. Optom. Vis. Sci. 2021, 98, 901–913. [Google Scholar] [CrossRef] [PubMed]
- De Novo Classification Request for Sensimed Triggerfish Contact Lens Sensor. Available online: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-device-senses-optimal-time-check-patients-eye-pressure (accessed on 26 April 2023).
- Mansouri, K.; Weinreb, R.N.; Liu, J.H.K. Efficacy of a contact lens sensor for monitoring 24-h intraocular pressure related patterns. PLoS ONE 2015, 10, e0125530. [Google Scholar] [CrossRef]
- Dunbar, G.E.; Shen, B.Y.; Aref, A. The Sensimed Triggerfish contact lens sensor: Efficacy, safety, and patient perspectives. Clin. Ophthalmol. 2017, 11, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Korb, C.; Herzog, N.; Vetter, J.M.; Elflein, H.; Keilani, M.M.; Pfeiffer, N. Tolerability of 24-hour intraocular pressure monitoring of a pressure-sensitive contact lens. J. Glaucoma 2013, 22, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Alayo, V.; Pérez-Torregrosa, V.T.; Clemente-Tomás, R.; Olate-Pérez, Á.; Cerdà-Ibáñez, M.; Gargallo-Benedicto, A.; Barreiro-Rego, A.; Duch-Samper, A. Efficacy of the SENSIMED Triggerfish® in the postoperative follow-up of PHACO-ExPRESS combined surgery. Arch. Soc. Española Oftalmol. 2017, 92, 372–378. (In English) [Google Scholar] [CrossRef]
- Vitish-Sharma, P.; Acheson, A.G.; Stead, R.; Sharp, J.; Abbas, A.; Hovan, M.; Maxwell-Armstrong, C.; Guo, B.; King, A.J. Can the SENSIMED Triggerfish® lens data be used as an accurate measure of intraocular pressure? Acta Ophthalmol. 2018, 96, e242–e246. [Google Scholar] [CrossRef] [PubMed]
- Rabensteiner, D.F.; Rabensteiner, J.; Faschinger, C. The influence of electromagnetic radiation on the measurement behaviour of the triggerfish® contact lens sensor. BMC Ophthalmol. 2018, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, X.; Li, X.; Yang, C.; Zhang, T.; Wu, Q.; Liu, D.; Lin, H.; Chen, W.; Hu, N.; et al. Wearable and implantable intraocular pressure biosensors: Recent progress and future prospects. Adv. Sci. 2021, 8, 2002971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, L.; Weinreb, R.N.; Cheng, H. Wearable electronic devices for glaucoma monitoring and therapy. Mater. Des. 2021, 212, 110183. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef]
- Ku, M.; Kim, J.; Won, J.-E.; Kang, W.; Park, Y.-G.; Park, J.; Lee, J.-H.; Cheon, J.; Lee, H.H.; Park, J.-U. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 2020, 6, eabb2891. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Du, X. Structurally coloured contact lens sensor for point-of-care ophthalmic health monitoring. J. Mater. Chem. B 2020, 8, 3519–3526. [Google Scholar] [CrossRef]
- Ye, Y.; Ge, Y.; Zhang, Q.; Yuan, M.; Cai, Y.; Li, K.; Li, Y.; Xie, R.; Xu, C.; Jiang, D.; et al. Smart contact lens with dual-sensing platform for monitoring intraocular pressure and matrix metalloproteinase-9. Adv. Sci. 2022, 9, e2104738. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, Y.; Lu, F.; Zhai, L.; Wu, H.; Chen, Z.; Wang, C.; Zhu, X.; Xie, Y.; Cai, P.; et al. Contact Lens Sensor with Anti-jamming Capability and High Sensitivity for Intraocular Pressure Monitoring. ACS Sens. 2023, 8, 2691–2701. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, Z.; Wu, X.; Gou, H.; Zhang, Y.; Ning, X.; Li, W.; Yao, Z.; Wang, Y.; Pei, W.; et al. High-sensitive microfluidic contact lens sensor for intraocular pressure visualized monitoring. Sens. Actuators A Phys. 2023, 354, 114250. [Google Scholar] [CrossRef]
- Jiang, N.; Montelongo, Y.; Butt, H.; Yetisen, A.K. Microfluidic contact lenses. Small 2018, 14, e1704363. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yao, H.; Zhao, G.; Ameer, G.A.; Sun, W.; Yang, J.; Mi, S. Flexible, wearable microfluidic contact lens with capillary networks for tear diagnostics. J. Mater. Sci. 2020, 55, 9551–9561. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, G.; Wang, A.; Sun, W.; Mi, S. Pressure-Triggered Microfluidic Contact Lens for Ocular Drug Delivery. ACS Appl. Polym. Mater. 2022, 4, 7290–7299. [Google Scholar] [CrossRef]
- Moreddu, R.; Elsherif, M.; Adams, H.; Moschou, D.; Cordeiro, M.F.; Wolffsohn, J.S.; Vigolo, D.; Butt, H.; Cooper, J.M.; Yetisen, A.K. Integration of paper microfluidic sensors into contact lenses for tear fluid analysis. Lab Chip 2020, 20, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kim, K.; Kim, H.J.; Meyer, D.; Park, W.; Lee, S.A.; Dai, Y.; Kim, B.; Moon, H.; Shah, J.V.; et al. Smart soft contact lenses for continuous 24-hour monitoring of intraocular pressure in glaucoma care. Nat. Commun. 2022, 13, 5518. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, P.; Yalcinkaya, A.D.; Ferhanoglu, O. Smart glasses to monitor intraocular pressure using optical triangulation. Opt. Commun. 2023, 546, 129752. [Google Scholar] [CrossRef]
- Zolfaghari, P.; Yalcinkaya, A.D.; Ferhanoglu, O. MEMS Sensor-Glasses Pair for Real-Time Monitoring of Intraocular Pressure. IEEE Photonics-Technol. Lett. 2023, 35, 887–890. [Google Scholar] [CrossRef]
- Marshall, L.L.; Hayslett, R.L.; Stevens, G.A. Therapy for open-angle glaucoma. Consult. Pharm.® 2018, 33, 432–445. [Google Scholar] [CrossRef]
- Digiuni, M.; Fogagnolo, P.; Rossetti, L. A review of the use of latanoprost for glaucoma since its launch. Expert Opin. Pharmacother. 2012, 13, 723–745. [Google Scholar] [CrossRef]
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New glaucoma medications: Latanoprostene bunod, netarsudil, and fixed combination netarsudil-latanoprost. Eye 2020, 34, 72–88. [Google Scholar] [CrossRef]
- Araie, M.; Sforzolini, B.S.; Vittitow, J.; Weinreb, R.N. Evaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24 h in healthy Japanese subjects. Adv. Ther. 2015, 32, 1128–1139. [Google Scholar] [CrossRef]
- Kawase, K.; Vittitow, J.L.; Weinreb, R.N.; Araie, M.; JUPITER Study Group Shigeru Hoshiai Setsuko Hashida Miki Iwasaki Kiyoshi Kano Kazuhide Kawase Takuji Kato Yasuaki Kuwayama Tomoyuki Muramatsu Masatada Mitsuhashi Sakae Matsuzaki Toru Nakajima Isao Sato Yuzuru Yoshimura. Long-term safety and efficacy of latanoprostene bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: The JUPITER study. Adv. Ther. 2016, 33, 1612–1627. [Google Scholar] [CrossRef] [PubMed]
- Demailly, P.; Allaire, C.; Bron, V.; Trinquand, C. Effectiveness and Tolerance of beta-Blocker/Pilocarpine Combination Eye Drops in Primary Open-Angle Glaucoma and High Intraocular Pressure. J. Glaucoma 1995, 4, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.J. Topical ophthalmic beta blockers: A comparative review. J. Ocul. Pharmacol. Ther. 1993, 9, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Dreischulte, T.; Lipworth, B.J.; Donnan, P.T.; Jackson, C.; Guthrie, B. Respiratory effect of beta-blocker eye drops in asthma: Population-based study and meta-analysis of clinical trials. Br. J. Clin. Pharmacol. 2016, 82, 814–822. [Google Scholar] [CrossRef]
- Reis, R.; Queiroz, C.F.; Santos, L.C.; Avila, M.P.; Magacho, L. A randomized, investigator-masked, 4-week study comparing timolol maleate 0.5%, brinzolamide 1%, and brimonidine tartrate 0.2% as adjunctive therapies to travoprost 0.004% in adults with primary open-angle glaucoma or ocular hypertension. Clin. Ther. 2006, 28, 552–559. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Adrenergic agonists and antagonists as antiglaucoma agents: A literature and patent review (2013–2019). Expert Opin. Ther. Patents 2019, 29, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Stoner, A.; Harris, A.; Oddone, F.; Belamkar, A.; Vercellin, A.C.V.; Shin, J.; Januleviciene, I.; Siesky, B. Topical carbonic anhydrase inhibitors and glaucoma in 2021: Where do we stand? Br. J. Ophthalmol. 2022, 106, 1332–1337. [Google Scholar] [CrossRef]
- Bacharach, J.; Dubiner, H.B.; Levy, B.; Kopczynski, C.C.; Novack, G.D.; AR-13324-CS202 Study Group. Double-masked, randomized, dose–response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology 2015, 122, 302–307. [Google Scholar] [CrossRef]
- Slota, C.; Sayner, R.; Vitko, M.; Carpenter, D.M.; Blalock, S.J.; Robin, A.L.; Muir, K.W.; Hartnett, M.E.; Sleath, B. Glaucoma patient expression of medication problems and nonadherence. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2015, 92, 537. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J. Improving compliance with glaucoma eye-drop treatment. Nurs. Times 1996, 92, 36–37. [Google Scholar] [PubMed]
- Shirley, M. Bimatoprost implant: First approval. Drugs Aging 2020, 37, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Sall, K.; DuBiner, H.; Benza, R.; Alster, Y.; Walker, G.; Semba, C.P.; Budenz, D.; Day, D.; Flowers, B.; et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: Results of a phase II randomized controlled study. Ophthalmology 2016, 123, 1685–1694. [Google Scholar] [CrossRef]
- Cvenkel, B.; Kolko, M. Devices and treatments to address low adherence in glaucoma patients: A narrative review. J. Clin. Med. 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Kesav, N.P.; Young, C.E.C.; Ertel, M.K.; Seibold, L.K.; Kahook, M.Y. Sustained-release drug delivery systems for the treatment of glaucoma. Int. J. Ophthalmol. 2021, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog. Retin. Eye Res. 2021, 82, 100901. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Mansoori, T.; Warjri, G.B.; Somarajan, B.I.; Bandil, S.; Gupta, V. Lasers in glaucoma. Indian J. Ophthalmol. 2018, 66, 1539. [Google Scholar] [CrossRef]
- Ha, J.Y.; Lee, T.H.; Sung, M.S.; Park, S.W. Efficacy and safety of intracameral bevacizumab for treatment of neovascular glaucoma. Korean J. Ophthalmol. 2017, 31, 538–547. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Stefan, C.; Batras, M.; De Simone, A.; Hosseini-Ramhormozi, J. Current Options for Surgical Treatment of Glaucoma. Rom. J. Ophthalmol. 2016, 59, 194–201. [Google Scholar]
- Zhu, Y.; Li, S.; Li, J.; Falcone, N.; Cui, Q.; Shah, S.; Hartel, M.C.; Yu, N.; Young, P.; de Barros, N.R.; et al. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics. Adv. Mater. 2022, 34, 2108389. [Google Scholar] [CrossRef]
- Zhu, Y.; Haghniaz, R.; Hartel, M.C.; Mou, L.; Tian, X.; Garrido, P.R.; Wu, Z.; Hao, T.; Guan, S.; Ahadian, S.; et al. Recent Advances in Bioinspired Hydrogels: Materials, Devices, and Biosignal Computing. ACS Biomater. Sci. Eng. 2021, 9, 2048–2069. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Hoare, T.R.; Iwata, N.G.; Behlau, I.; Dohlman, C.H.; Langer, R.; Kohane, D.S. A drug-eluting contact lens. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.V.; Kalout, P.; Pasquale, L.R.; Kohane, D.S.; Ciolino, J.B. Clinicians’ perspectives on the use of drug-eluting contact lenses for the treatment of glaucoma. Ther. Deliv. 2014, 5, 1077–1083. [Google Scholar] [CrossRef]
- Ding, X.; Ben-Shlomo, G.; Que, L. Soft contact lens with embedded microtubes for sustained and self-adaptive drug delivery for glaucoma treatment. ACS Appl. Mater. Interfaces 2020, 12, 45789–45795. [Google Scholar] [CrossRef]
- Soluri, A.; Hui, A.; Jones, L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom. Vis. Sci. 2012, 89, 1140–1149. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Next-generation contact lenses: Towards bioresponsive drug delivery and smart technologies in ocular therapeutics. Eur. J. Pharm. Biopharm. 2021, 161, 80–99. [Google Scholar] [CrossRef]
- Lai, C.F.; Shiau, F.J. Enhanced and Extended Ophthalmic Drug Delivery by pH-Triggered Drug-Eluting Contact Lenses with Large-Pore Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 18630–18638. [Google Scholar] [CrossRef]
- Brandt, J.D.; DuBiner, H.B.; Benza, R.; Sall, K.N.; Walker, G.A.; Semba, C.P.; Budenz, D.; Day, D.; Flowers, B.; Lee, S.; et al. Long-term safety and efficacy of a sustained-release bimatoprost ocular ring. Ophthalmology 2017, 124, 1565–1566. [Google Scholar] [CrossRef]
- Ruiz-Pomeda, A.; Villa-Collar, C. Slowing the progression of myopia in children with the MiSight contact lens: A narrative review of the evidence. Ophthalmol. Ther. 2020, 9, 783–795. [Google Scholar] [CrossRef]
- Lanier, O.L.; Christopher, K.G.; Macoon, R.M.; Yu, Y.; Sekar, P.; Chauhan, A. Commercialization challenges for drug eluting contact lenses. Expert Opin. Drug Deliv. 2020, 17, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.; Reddy, R.J.; Maheswaran, T.; Asokan, G.S.; Dany, A.; Anand, B. Theranostics: A treasured tailor for tomorrow. J. Pharm. Bioallied Sci. 2014, 6 (Suppl. S1), S6. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Theranostics: Are we there yet? Mol. Pharm. 2013, 10, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Mok, J.W.; Hong, S.H.; Jeong, S.H.; Choi, H.; Shin, S.; Joo, C.-K.; Hahn, S.K. Wireless theranostic smart contact lens for monitoring and control of intraocular pressure in glaucoma. Nat. Commun. 2022, 13, 6801. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, Q.; Liu, J.; Mo, J.; Li, X.; Yang, C.; Liu, Z.; Yang, J.; Jiang, L.; Chen, W.; et al. Intelligent wireless theranostic contact lens for electrical sensing and regulation of intraocular pressure. Nat. Commun. 2022, 13, 2556. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Sun, M.; Hua, M.; He, X. Bioinspired structural color sensors based on responsive soft materials. Curr. Opin. Solid State Mater. Sci. 2019, 23, 13–27. [Google Scholar] [CrossRef]
- Qin, M.; Sun, M.; Bai, R.; Mao, Y.; Qian, X.; Sikka, D.; Zhao, Y.; Qi, H.J.; Suo, Z.; He, X. Bioinspired hydrogel interferometer for adaptive coloration and chemical sensing. Adv. Mater. 2018, 30, e1800468. [Google Scholar] [CrossRef]
- Frenkel, I.; Hua, M.; Alsaid, Y.; He, X. Self-Reporting Hydrogel Sensors Based on Surface Instability-Induced Optical Scattering. Adv. Photonics Res. 2021, 2, 2100058. [Google Scholar] [CrossRef]
- Choi, J.; Hua, M.; Lee, S.Y.; Jo, W.; Lo, C.; Kim, S.; Kim, H.; He, X. Hydrocipher: Bioinspired dynamic structural color-based cryptographic surface. Adv. Opt. Mater. 2020, 8, 1901259. [Google Scholar] [CrossRef]
- Sun, M.; Bai, R.; Yang, X.; Song, J.; Qin, M.; Suo, Z.; He, X. Hydrogel interferometry for ultrasensitive and highly selective chemical detection. Adv. Mater. 2018, 30, 1804916. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shean, R.; Yu, N.; Guntipally, S.; Nguyen, V.; He, X.; Duan, S.; Gokoffski, K.; Zhu, Y.; Xu, B. Advances and Challenges in Wearable Glaucoma Diagnostics and Therapeutics. Bioengineering 2024, 11, 138. https://doi.org/10.3390/bioengineering11020138
Shean R, Yu N, Guntipally S, Nguyen V, He X, Duan S, Gokoffski K, Zhu Y, Xu B. Advances and Challenges in Wearable Glaucoma Diagnostics and Therapeutics. Bioengineering. 2024; 11(2):138. https://doi.org/10.3390/bioengineering11020138
Chicago/Turabian StyleShean, Ryan, Ning Yu, Sourish Guntipally, Van Nguyen, Ximin He, Sidi Duan, Kimberly Gokoffski, Yangzhi Zhu, and Benjamin Xu. 2024. "Advances and Challenges in Wearable Glaucoma Diagnostics and Therapeutics" Bioengineering 11, no. 2: 138. https://doi.org/10.3390/bioengineering11020138
APA StyleShean, R., Yu, N., Guntipally, S., Nguyen, V., He, X., Duan, S., Gokoffski, K., Zhu, Y., & Xu, B. (2024). Advances and Challenges in Wearable Glaucoma Diagnostics and Therapeutics. Bioengineering, 11(2), 138. https://doi.org/10.3390/bioengineering11020138