Acquisition Devices for Fetal Phonocardiography: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
- Eligible works must be related to fetal monitoring. Identified keywords: “fetal”, “pregnancy”, “fetus”, “prenatal”, “antenatal”, “foetal”, “foetus”;
- Eligible works must be related to heart sounds. Identified keywords: “phonocardiography”, “heart sounds”, “FPCG”, “PCG”, “heart murmur”, “acoustic cardiography”, “auscultation”;
- Eligible works must provide technical information concerning the device design. Identified keywords: “hardware”, “device”, “system”, “recording”, “acquisition”, “microphone”.
2.2. Selection of Studies
- Written in a language other than English;
- Belonging to one of the following document types: guidelines, conference collection, and editorial.
- Analysis of the title;
- Analysis of the abstract (if available);
- Analysis of the full text (if available).
- Non-technical studies (i.e., studies using fetal auscultation for clinical purposes, without focusing on the device) were excluded;
- Studies describing devices not supporting the recording of heart sounds were excluded;
- Studies describing devices for the recording of heart sounds in children or adults were excluded;
- Studies focusing on signal processing, not providing a description of the device, were excluded.
2.3. Data Charting and Synthesis
- Device: number, type and position of microphone sensors, availability of other types of sensors, type of sensor–skin interface (head of the sensor), fasten means to the maternal abdomen, system architecture, hardware characteristics (filters, gain, ADC dynamics, sampling frequency, type of processor, memory, power supply);
- Signal processing: method for denoising, method for FHR estimation, other processing;
- Validation: goal of the validation study, comparison against a gold standard, size of the sample population, gestational age, performances.
- Manufacturer and commercial name;
- Availability on the market at the search date;
- CE mark according to the Medical Device Regulation 2017/745;
- Whether the device is specifically designed for fetal application or not.
3. Results
3.1. Literature Search
3.2. Hardware Characteristics
3.3. Signal Processing and Clinical Validation
| Study | Sensors | System | Hardware Characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dev./ Ref. | Year | Class | N | Type | Position | Other | Head Type | Arch. | Fasten Means | Filters | Gain | ADC | SF | Processor | Memory | Power Supply |
| RP1/ [22,23] | 1993 | A | 3–7 | Piezo-electric | Depends on fetus position | - | H4 | T1 | Belt | 20 Hz–55 Hz | Y | - | - | - | - | - |
| RP2/ [24] | 1953 | A | 1 | Piezo-electric | - | - | H2 | T1 | - | - | >105 | - | - | - | - | - |
| RP3/ [25] | 1964 | I | 1 | Sonic transd. | Intrauterine | - | - | T1 | - | - | Y | - | - | - | - | - |
| RP4/ [26] | 2019 | SC | 1 | Electret | - | - | H1 | T7 | - | N | N | N | 16 kHz | N | N | Via phone |
| RP5/ [27] | 2019 | SC | 1 | Electret | Depends on fetus position | - | H1 | T6 | Adhesive tape | - | - | 16 bit | 44.1 kHz | - | - | - |
| RP6/ [28] | 2019 | SC | 1 | Electret | - | - | H3 | T9 | - | 15 Hz–3 kHz | 9 | 16 bit | 48 kHz | CSR8670/master control chip | 16 Mb flash memory | - |
| RP7/ [29,30,31] | 2018 | SC | 1–3 | Electret | Depends on fetus position | - | H1 H2 | T8 | Handheld | 16 Hz–20 kHz | Y | 16 bit | 5 kHz | ARM Cortex-M4 | - | Battery |
| RP8/ [32,33] | 2018 | SC | 1 | Piezo-electric | - | - | H2 | T9 | - | - | 12 | - | 44.1 kHz | - | - | Battery |
| RP9/ [34] | 2017 | SC | 1 | Electret | - | - | H3 | T10 | - | DC to 200 Hz | Y | - | - | Arduino Uno R3 (ATMega328) | - | Battery |
| RP10/ [35] | 2017 | SC | 1 | - | 3 cm left/right, 1 cm up/down the navel | - | H2 | T9 | - | - | Y | - | - | - | - | - |
| RP11/ [36] | 2012 | SC | 1 | Condenser | - | - | H1 | T11 | - | - | Y | - | - | - | - | - |
| RP12/ [37,38] | 2008 | SC | 1 | Piezo-electric | - | - | H1 | T8 | - | DC to 70 Hz | - | - | 8 kHz | - | - | - |
| RP13/ [39,40] | 2006 | SC | 1 | Electret | Beside CTG | - | H1 | T10 | - | DC to 110 Hz | Adj. | 16 bit | 2 kHz | Intel XScale 624 MHz | 128 MB Flash ROM + 64 MB SDRAM | Battery |
| RP14/ [41] | 2003 | SC | 1 | - | - | - | H3 | T8 | - | - | - | 16 bit | 1 kHz | - | - | - |
| RP15/ [42] | 2000 | SC | 1 | - | - | - | H2 | T5 | - | - | - | - | - | - | - | - |
| RP16/ [43,44,45,46,47] | 1986 | SC | 1 | Piezo-electric (TAPHO sensor) | Depends on fetus position | - | H3 | T3 | Double-sided adhesive discs | - | - | - | - | - | - | - |
| RP17/ [48] | 2020 | MC | 3 | MEMS | 100 mm triangle | - | H5 | T7 | - | - | Y | 16 bit | 5 kHz | ARM Micro Control Unit | - | - |
| RP18/ [11,49] | 2017 | MC | 4 | Piezo-electric | 160 mm square around the navel | - | H5 | T8 | 3D-printed harness | - | - | 16 bit | 1 kHz | - | - | - |
| RP19/ [50] | 2023 | MC + MS | 3 | Condenser | Depends on fetus position | 3 electrodes for EHG recording | H4 | T9 | Elastic belt | - | - | 32 bit | - | Shakti Parashu processor (Arty A7-100T FPGA) | 128 MB RAM | Battery |
| RP20/ [51,52] | 2000 | MC + MS | 6 | Sound guide | - | Microphone for mPCG | H3 | T8 | Strap | DC to 200 Hz | 1 to 8 | 12 bit | 1024 Hz | - | - | - |
| RP21/ [53,54] | 2018 | MS | 1 | Piezo-electric | - | Microphone for mPCG | H3 | T6 | - | DC to 80 Hz | AGC | - | 1 kHz | - | Y | Battery |
| RP22/ [55,56,57,58,59] | 2017 | MS | 1 | Fiber optic | - | Microphone for mPCG | H5 | T8 | Self-adhesive straps | 0.5 Hz–400 Hz | 1 to 50 | 16 bit | 1 kHz | NI USB 6210 card | - | - |
| RP23/ [60,61] | 2013 | MS | 1 | - | - | Microphone for ambient noise | H2 | T7 | - | - | Adj. | 24 bit | 2 kHz | MSP430 microcontroller by TI | 32 GB flash mem. | Battery |
| RP24/ [62] | 2007 | MS | 1 | Piezo-electric | - | Microphone for ambient noise | H1 | T8 | - | DC to 70 Hz | Y | - | - | - | - | Battery |
| RP25/ [63,64] | 2001 | MS | 1 | Electret | - | Microphone for ambient noise | H1 | T4 | Handheld | - | Y | 16 bit | 11,025 Hz | - | - | Battery |
| RP26/ [65,66] | 1991 | MS | 1 | Inductive (INPHO) | Few cm below the navel | Electrode for FECG/mECG | H3 | T4 | Double-sided tape | DC to 200 Hz | Y | 12 bit | 640 Hz | Olivetti M290 microcomputer | 20 MB | - |
| Study | Commercial Information | Sensors | System | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dev./ Ref. | Year | Class | Manufacturer | Commercial Name | On Market | CE Mark | N | Type | Position | Other | Head Type | Arch. | Fasten Means |
| CD1/ [67] | 1941 | A | Cambridge Instrument Inc. (London, UK) | Electrocardiograph-stethograph | N | - | 1 | - | Depends on fetus position | Electrodes for FECG | - | T1 | Rubber strap |
| CD2/ [68] | 1970 | A | Jaeger Laboratories (Columbus, OH, USA) | Model 3 solid-state amplifier | N | - | 6 | Generic mic. | - | - | - | T1 | Rubber strap |
| CD3/ [69] | 1993 | A | Smith Kline Instruments (Sunnyvale, CA, USA) | Smith Kline Model EKS-1 | N | - | 1 | - | - | - | - | - | - |
| CD4/ [70] | 2020 | SC | Ayu Devices (Mumbai, India) | Ayusynk digital stethoscope | Y | N | 1 | - | - | N | H2 | T3 | Handheld |
| CD5/ [70] | 2020 | SC | TE Connectivity (Schaffausen, Switzerland) | Contact microphone CM-01b | Y | N | 1 | Piezo-electric | - | - | H3 | T3 | - |
| CD6/ [71] | 2015 | SC | GS Technology (Seoul, Republic of Korea) | JABES | Y | Y | 1 | - | Lower abdomen, follows analog auscultation | N | H2 | T4 | Handheld |
| CD7/ [70,72,73] | 2022 | MS | BIOPAC Systems Inc. (Goleta, CA, USA) | MP36 system + SS30LA/SS30L | Y | Y | 1 | Piezo-electric | - | Electrodes/microphones can be added | H2 | T4 | Handheld |
| CD8/ [74,75,76,77] | 2018 | MS | ADInstruments (Sydney, NSW, Australia) | Cardio Microphone MLT201 + Powerlab acquisition system | Y | Y | 1 | Electret | - | Electrodes/microphones can be added | H2 | T8 | Adhesive tape |
| Study | Signal Processing | Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dev | Ref | Year | Class | Denoising | FHR Estimation | Other | Goal | Gold Standard | Pop. Size | Gest. Age (Weeks) | Results |
| RP1 | [22] | 1990 | A | - | - | Power spectrum density | Sound | - | - | 35–39 | Qualitative appraisal |
| [23] | 1993 | - | Y | Heart sound detection (linear prediction) | Sound | CTG | 16 | - | In vitro: calibration error = ±1.3 dB to ±2.5 dB. In vivo: qualitative appraisal. | ||
| RP2 | [24] | 1953 | A | - | - | - | Sound | - | - | - | Qualitative appraisal |
| RP3 | [25] | 1964 | I | - | - | - | - | - | - | - | - |
| RP4 | [26] | 2019 | SC | Butterworth BPF 20–200 Hz + WT | Cyclostationary process in frequency domain | - | FHR | Doppler | 10 | 36–39 | Agreement = 96% (Bland–Altman) |
| RP5 | [27] | 2019 | SC | - | - | - | Sound | Electronic steth. | 5 | >37 | Increased SNR, decreased loss due to artifacts |
| RP6 | [28] | 2019 | SC | - | - | Play the sound | Sound | - | - | - | - |
| RP7 | [29] | 2018 | SC | - | Y | - | FHR | CTG | 1 | - | Qualitative appraisal |
| [30] | 2018 | BPF 0.5–70 Hz | Envelope-based | - | FHR | CTG | 1 | 34 | Measured FHR = 133 bpm vs. CTG = 120–150 bpm | ||
| [31] | 2019 | FIR BPF 20–110 Hz | - | PSD | Sound PSD | - | 1 | 34 | Qualitative appraisal | ||
| RP8 | [32] | 2018 | SC | BPF 10–400 Hz + SVR, EMD, adaptive LMS, Wavelet | - | - | Sound | - | - | - | SVR best denoising; qualitative appraisal |
| [33] | 2021 | SVR, EMD, adaptive LMS, WT | Time windowing | - | FHR | - | - | - | SVR best denoising; qualitative appraisal | ||
| RP9 | [34] | 2017 | SC | - | Peak detection | - | FHR | Electronic stethoscope | 10 | 13–38 | Bias = −1.2% to 1.4%, Tolerance = ±5 bpm |
| RP10 | [35] | 2017 | SC | - | Y | - | FHR | - | 7 | - | Separation FPCG/MPCG possible in 70% of cases |
| RP11 | [36] | 2012 | SC | DWT | Envelope-based | - | FHR | Doppler | 15 | 28–38 | Acc = 98% |
| RP12 | [37] | 2008 | SC | FIR BPF 20–200 Hz | Manual labelling S1 | - | Sound FHR | Doppler | 21 | 36–40 | Clear sound in 15/21 cases, Acc = 98% |
| [38] | 2011 | DWT | - | Heart sounds segmentation (envelope-based) + CWT | Time frequency | - | 18 | - | Qualitative appraisal | ||
| RP13 | [39,40] | 2006 | SC | Butterworth IIR HPF 35 Hz | Envelope-based | Confidence factor estimation | FHR | CTG | 41 | 37–38 | Agreement = 75%; qualitative appraisal |
| RP14 | [41] | 2003 | SC | FIR BPF 10–50 Hz | Envelope-based | Heart sounds detection | FHR | Monitor | 2 | - | - |
| RP15 | [42] | 2000 | SC | - | Adaptive correlation | - | FHR | - | 10 | - | Qualitative appraisal |
| RP16 | [43] | 1986 | SC | HPF 50 Hz | - | - | Sound | - | 140 | 20–29 | Heart sounds detection; on qualitative appraisal: 0% in <20 w, 22% in 20–24 w, 83% in 25–29 w, 100% in >30 w |
| [44] | 1986 | HPF 50 Hz | - | - | - | - | - | - | - | ||
| [45] | 1986 | HPF 40 Hz | - | Manual labelling | Systolic time vs. breathing/moving | FECHO | 12 | 28–41 | R = 0.86 Systolic time vs. fetal breathing | ||
| [46] | 1989 | BPF 45 Hz to 65 Hz | Full-wave rectifier + variable comb filtering | - | FHR | Scalp FECG | - | - | AE < 3% | ||
| [47] | 1989 | HPF 20 Hz | - | Heart sounds segmentation (peak detection) | Systolic time | - | - | - | - | ||
| RP17 | [48] | 2020 | MC | BPF (not spec.) + DWT | Y (not specified) | Fetal heart localization (CNN on power images) | FHR fetal position | Monitor, B-scan US | 16 | - | FHR:AE = 4.3 bpm, Fetal location: Acc = 100% (tolerance 33 mm) |
| RP18 | [11] | 2017 | MC | Notch filter 50 Hz | Peak detection | FPCG/mPCG separation (BSS) | FHR | CTG | 20 | - | AE = −0.21 bpm (2SD = ±3 bpm) |
| [49] | 2018 | WTST-NST | Peak detection | FPCG/mPCG/mResp separation (refBSS) | FHR | FECG, Doppler | 15 | 33–40 | R = 0.96 vs. FECG | ||
| RP19 | [50] | 2023 | MC + MS | - | Y | - | FHR | Doppler | 10 | 28–40 | Qualitative appraisal |
| RP20 | [51] | 2000 | MC + MS | LPF 188.1 Hz | Y | - | FHR | - | 1 | 37 | Qualitative appraisal |
| [52] | 2003 | LPF 188.1 Hz | - | Power spectrum analysis | Sound PSD | - | 1 | 37 | Qualitative appraisal | ||
| RP21 | [53] | 2018 | MS | - | Y | - | FHR | Monitor | 50 | - | AE ≤ 2 bpm |
| [54] | 2018 | BPF | Envelope-based | - | FHR | Monitor | 50 | - | AE ≤ 2 bpm, agreement on fetal heart status = 100% | ||
| RP22 | [55] | 2017 | MS | - | Y | FPCG/mPCG separation (adaptive + nLMS) | FHR Sound SNR | - | 8 | 36–42 | Qualitative appraisal |
| [56] | 2017 | - | - | FPCG/mPCG separation (adaptive + nLMS) | Sound SNR | - | - | - | Tested on simulated data | ||
| [57] | 2017 | - | - | FPCG/mPCG separation (RLS) | Sound SNR | - | 5 | - | Qualitative appraisal | ||
| [58] | 2017 | - | Peak detection | FPCG/mPCG separation (adaptive + LMS/nLMS) | FHR Sound SNR | - | 10 | 35–42 | Qualitative appraisal | ||
| [59] | 2018 | - | - | FPCG/mPCG separation (LMS/RLS) | Sound SNR | - | - | - | Tested on simulated data | ||
| RP23 | [60] | 2013 | MS | - | - | - | - | - | - | - | - |
| [61] | 2014 | Computational auditory scene analysis | Adaptive matching | - | FHR | Doppler | 8 | 37–40 | AE < 10% | ||
| RP24 | [62] | 2007 | MS | Adaptive filter | Y | - | FHR | CTG | 16 | 36–40 | Acc = 97.95% |
| RP25 | [63] | 2001 | MS | DWT + BPF 35–200 Hz + adaptive cross-channel cancellation | - | - | Sound | - | 3 | - | Qualitative appraisal |
| [64] | 2003 | BPF 35–200 Hz + DWT | Envelope + cross-correlation | - | FHR | CTG | 9 | 28–40 | 2:Acc > 90%; 3:Acc > 80%; 2:Acc > 70%; 2:Acc < 70% | ||
| RP26 | [65] | 1991 | MS | Adaptive filter | - | - | SNR | - | 5 | - | Qualitative appraisal |
| [66] | 1991 | BPF 40–80 Hz | Y | Fetal movement identification | FHR, fetal moving | FECHO, IUP | 6 | 36–39 | SNR = 96 dB in lab SNR = 78 dB in real data | ||
| CD1 | [67] | 1941 | A | - | - | Manual labelling | Alive fetus | - | 40 | 32–40 | Acc = 100% |
| CD2 | [68] | 1970 | MC | - | - | - | Sound | - | - | - | - |
| CD3 | [69] | 1993 | SC | - | - | - | - | - | - | - | - |
| CD4 | [70] | 2020 | SC | - | - | - | SNR | - | - | - | - |
| CD5 | [70] | 2020 | SC | - | - | - | SNR | - | - | - | - |
| CD6 | [71] | 2015 | SC | - | Y | Single-channel BSS (EMD + NNMF) | FHR | FECHO | 50 | 30–40 | Acc = 96% |
| CD7 | [72] | 2001 | MS | - | Multiresolution analysis + Hilbert transform | Estimation of the HRV signal and extraction of variability indexes | FHR and its analysis | FECG | 11 | 28–41 | MS2 was found as the only reliable time reference |
| [70] | 2020 | - | - | - | SNR | - | - | - | - | ||
| [73] | 2022 | BPF (adjustable frequencies) | Envelope + cross-correlation | - | FHR | Manual | 99 | 30–40 | Manual annotation tested on public dataset with AE = 0.85 bpm. In vivo, 60/99 recordings with sufficient quality, MAE = 7.54, PPV = 87% | ||
| CD8 | [74] | 2018 | MS | BPF 20–250 Hz | Envelope-based | - | FHR | CTG | 9 | 24–39 | R = 0.64 to 0.84 |
| [75] | 2019 | BPF 20–200 Hz | Spectrogram + NNMF | - | FHR | CTG | 4 | 38–39 | R ≈ 0.9 | ||
| [76] | 2022 | BPF 20–250 Hz | Spectrogram + NNMF | - | FHR | CTG | 38 | >37 | 25/38 suitable recordings; qualitative appraisal; average agreement = 75% | ||
| [77] | 2023 | FIR BPF 20–200 Hz | Spectrogram + NNMF + HMM + modified Viterbi algorithm | - | FHR | CTG | 6 | 38–39 | Modified Viterbi algorithm reduces confusion with maternal HR to less than 1% | ||
4. Discussion
4.1. General Overview
4.2. System Architecture and Use of Sensors
4.3. Signal Processing
- Digital filtering (25 studies). A detailed analysis of the reported frequency bandwidth is proposed in Figure 11. As already discussed in Section 4.2, the use of digital filtering is typically devoted to limiting the bandwidth of the signal to the typical bandwidth of the two main fetal heart sounds (20 Hz to 120 Hz);
- Wavelet decomposition (nine studies);
- Adaptive filtering (four studies);
- Other methods including support vector regression (two studies), empirical mode decomposition (two studies), computational auditory scene analysis (one study) and adaptive cross-channel cancellation (one study).
- Separation of fetal and maternal heart sounds by means of adaptive filtering and least mean square (five studies) or blind source separation (two studies);
- Segmentation of the first and second heart sounds by means of envelope-based peak detection and cardiac time intervals estimation (four studies);
- Power spectrum analysis (three studies);
- Time-frequency analysis using spectrograms (one study);
- Identification of fetal movements by means of filtering (one study);
- Heart rate variability estimation and analysis (one study);
- Single-channel blind source separation using a combination of empirical mode decomposition and non-negative matrix factorization (one study);
- Fetal heart localization using convolutional neural networks on images built on top of the power spectrum (one study)
- Confidence factor estimation (one study)
4.4. Validation
4.5. Comparison against State-of-the-Art Reviews
| Ref. | Year | Focus | Type/ Guideline | FPCG Physiology and Modeling | FPCG Acquisition Systems | Signal Processing | Clinical Validation |
|---|---|---|---|---|---|---|---|
| Kovács et al. [19] | 2011 | Overview of FPCG works on the applied signal processing methods for identification of sound components | - | YES | Briefly introduced | Generally treated | Generally treated |
| Adithya et al. [20] | 2017 | Trends in data collection, signal processing techniques, and synthesis models related to FPCG | - | YES | Briefly introduced | Systematically analyzed | Related to signal processing |
| Proposed review | 2024 | Investigation and trends of the FPCG acquisition systems | Scoping/ PRISMA | NO | Systematically analyzed | Analyzed in connection to the acquisition system | Related to acquisition system |
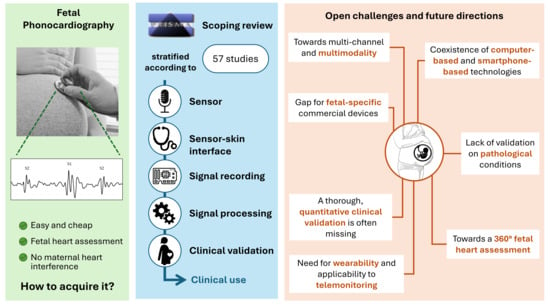
4.6. Open Challenges
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Who Mortality Database. Available online: https://platform.who.int/mortality/themes/theme-details/mdb/communicable-maternal-perinatal-and-nutritional-conditions (accessed on 11 March 2024).
- Alkema, L.; Chou, D.; Hogan, D.; Zhang, S.; Moller, A.-B.; Gemmill, A.; Fat, D.M.; Boerma, T.; Temmerman, M.; Mathers, C.; et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: A systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016, 387, 462–474. [Google Scholar] [CrossRef]
- Hogan, M.C.; Foreman, K.J.; Naghavi, M.; Ahn, S.Y.; Wang, M.; Makela, S.M.; Lopez, A.D.; Lozano, R.; Murray, C.J. Maternal mortality for 181 countries, 1980–2008: A systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010, 375, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pregnancy, Childbirth, Postpartum and Newborn Care: A Guide for Essential Practice; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154935-6. [Google Scholar]
- Nasiri, S.; Khosravani, M.R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sens. Actuators A Phys. 2020, 312, 112105. [Google Scholar] [CrossRef]
- WHEC Practice Bulletin and Clinical Management Guidelines for Healthcareproviders, Intrapartum Electronic Fetal Monitoring, Women’s Health and Education Center (WHEC). Available online: http://www.womenshealthsection.com/content/obs/obs028.php3 (accessed on 11 March 2024).
- Ayres-de-Campos, D.; Bernardes, J. Twenty-five years after the FIGO guidelines for the use of fetal monitoring: Time for a simplified approach? Int. J. Gynecol. Obstet. 2010, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A.; Miller, L.A. Electronic fetal heart rate monitoring: Applying principles of patient safety. Am. J. Obstet. Gynecol. 2012, 206, 278–283. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Child Health and Human Development Research Planning Workshop. Electronic fetal heart rate monitoring: Research guidelines for interpretation. J. Obstet. Gynecol. Neonatal Nurs. 1997, 26, 635–640. [Google Scholar] [CrossRef]
- Downs, T.; Zlomke, E. Fetal heart rate pattern notification guidelines and suggested management algorithm for intrapartum electronic fetal heart rate monitoring. Perm J. 2007, 4, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A.; Al Awar, S.; Balayah, Z.H.; Hadjileontiadis, L.J.; Khandoker, A.H. A Comparative Study on Fetal Heart Rates Estimated from Fetal Phonography and Cardiotocography. Front. Physiol. 2017, 8, 764. [Google Scholar] [CrossRef]
- Rychik, J.; Ayres, N.; Cuneo, B.; Gotteiner, N.; Hornberger, L.; Spevak, P.J.; Van Der Veld, M. American society of echocardiography guidelines and standards for performance of the fetal echocardiogram. J. Am. Soc. Echocardiogr. 2004, 17, 803–810. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Sameni, R.; Clifford, G.D. A Review of Fetal ECG Signal Processing Issues and Promising Directions. Open Pacing Electrophysiol. Ther. J. 2010, 3, 4. [Google Scholar] [CrossRef]
- Peters, M.; Crowe, J.; Piéri, J.-F.; Quartero, H.; Hayes-Gill, B.; James, D.; Stinstra, J.; Shakespeare, S. Monitoring the fetal heart non-invasively: A review of methods. J. Perinat. Med. 2001, 29, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, Y.; Zhou, Z.; Lin, L.; Zeng, Y.; Gao, X. Research of fetal ECG extraction using wavelet analysis and adaptive filtering. Comput. Biol. Med. 2013, 43, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Solum, T. Antenatal Cardiotocography: Methods, interpretation and clinical application. Acta Obstet. Gynecol. Scand. 1980, 59, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, T.; Qiu, T.; Park, Y. Fetal Heart Rate Monitoring from Phonocardiograph Signal Using Repetition Frequency of Heart Sounds. J. Electr. Comput. Eng. 2016, 2016, 2404267. [Google Scholar] [CrossRef]
- Kovács, F.; Horváth, C.; Balogh, Á.T.; Hosszú, G. Fetal phonocardiography—Past and future possibilities. Comput. Methods Programs Biomed. 2011, 104, 19–25. [Google Scholar] [CrossRef]
- Chetlur Adithya, P.; Sankar, R.; Moreno, W.A.; Hart, S. Trends in fetal monitoring through phonocardiography: Challenges and future directions. Biomed. Signal Process. Control 2017, 33, 289–305. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Stoughton, J.W.; Weber, G.N.; Pretlow, R.A. Fetal heart rate estimation via adaptive least mean square linear prediction methods. In Proceedings of the IEEE Proceedings on Southeastcon, New Orleans, LA, USA, 1–4 April 1990; pp. 260–264. [Google Scholar]
- Zuckerwar, A.J.; Pretlow, R.A.; Stoughton, J.W.; Baker, D.A. Development of a piezopolymer pressure sensor for a portable fetal heart rate monitor. IEEE Trans. Biomed. Eng. 1993, 40, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.L.; Wood, M.C. The Amplification and Recording of Fœtal Heart Sounds. Proc. R. Soc. Med. 1953, 46, 85–91. [Google Scholar] [CrossRef]
- Richeson, W.E.; Hagan, W.K.; Stander, R.W.; Barden, T.P.; Thompson, J.F. A transducer for intra-uterine fetal phonocardiography. Med. Electron. Biol. Eng. 1964, 2, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ye, S.; Huang, Z.; Jiaerken, D.; Zhao, S.; Zhang, L.; Wu, J. A Noninvasive Continuous Fetal Heart Rate Monitoring System for Mobile Healthcare Based on Fetal Phonocardiography. In Internet of Things; Springer: Cham, Switzerland, 2019; pp. 191–204. [Google Scholar]
- Charlier, P.; Herman, C.; Rochedreux, N.; Logier, R.; Garabedian, C.; Debarge, V.; Jonckheere, J. De AcCorps: A low-cost 3D printed stethoscope for fetal phonocardiography. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 52–55. [Google Scholar]
- Huang, H.; Yang, D.; Yang, X.; Lei, Y.; Chen, Y. Portable multifunctional electronic stethoscope. In Proceedings of the 2019 IEEE 3rd Information Technology, Networking, Electronic and Automation Control Conference (ITNEC), Chengdu, China, 15–17 March 2019; pp. 691–694. [Google Scholar]
- Kolarik, J.; Soustek, L.; Martinek, R. Examination and Optimization of the Fetal Heart Rate Monitor: Evaluation of the effect influencing the measuring system of the Fetal Heart Rate Monitor. In Proceedings of the 2018 IEEE 20th International Conference on E-Health Networking, Applications and Services (Healthcom), Ostrava, Czech Republic, 17–20 September 2018; pp. 1–4. [Google Scholar]
- Kolarik, J.; Golembiovsky, M.; Docekal, T.; Kahankova, R.; Martinek, R.; Prauzek, M. A Low-cost Device for Fetal Heart Rate Measurement. IFAC-PapersOnLine 2018, 51, 426–431. [Google Scholar] [CrossRef]
- Kahankova, R.; Kolarik, J.; Martinek, R.; Durikova, A. A Comparative Analysis of Fetal Phonocardiograph Acoustical Performance. IFAC-PapersOnLine 2019, 52, 514–519. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, J.; Li, X.; Liu, Z.; Su, F. Adaptive SVR Denoising Algorithm for Fetal Monitoring System. In Proceedings of the 2018 10th International Conference on Wireless Communications and Signal Processing (WCSP), Hangzhou, China, 18–20 October 2018; pp. 1–5. [Google Scholar]
- Wei, J.; Wang, Z.; Xing, X. A Wireless High-Sensitivity Fetal Heart Sound Monitoring System. Sensors 2020, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Shabry, N.A.N.B.M.; Singh, O.P.; Sardana, P.; Hisham, R.B.; Malarvili, M.B. Home based fetal heart rate monitor. Int. J. Appl. Eng. Res. 2017, 12, 813–817. [Google Scholar]
- Sipka, G.; Szabó, T.; Zölei-Szénási, R.; Vanya, M.; Jakó, M.; Nagy, T.D.; Fidrich, M.; Bilicki, V.; Borbás, J.; Bitó, T.; et al. Monitoring of Fetal Heart Rate via iPhone. In Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer: Cham, Switzerland, 2017; pp. 492–496. [Google Scholar]
- Chourasia, V.S.; Tiwari, A.K. Implementation of foetal e-health monitoring system through biotelemetry. Int. J. Electron. Healthc. 2012, 7, 36–52. [Google Scholar] [CrossRef]
- Chourasia, V.; Mittra, A. Passive acoustic signal acquisition system for non-invasive fetal heart sound monitoring applications. Internet J. Med. Technol. 2009, 5, 59056894. [Google Scholar]
- Chourasia, V.S.; Tiwari, A.K.; Gangopadhyay, R. Time-Frequency Characterization of Fetal Phonocardiographic Signals Using Wavelet Scalogram. J. Mech. Med. Biol. 2011, 11, 391–406. [Google Scholar] [CrossRef]
- Chen, J.; Phua, K.; Song, Y.; Shue, L. Fetal Heart Signal Monitoring with Confidence Factor. In Proceedings of the 2006 IEEE International Conference on Multimedia and Expo, Toronto, ON, Canada, 9–12 July 2006; pp. 1937–1940. [Google Scholar]
- Chen, J.; Phua, K.; Song, Y.; Shue, L. A portable phonocardiographic fetal heart rate monitor. In Proceedings of the 2006 IEEE International Symposium on Circuits and Systems, Kos, Greece, 21–24 May 2006; p. 4. [Google Scholar]
- Godinez, M.; Jimenez, A.; Ortiz, R.; Pena, M. On-line fetal heart rate monitor by phonocardiography. In Proceedings of the Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Cancun, Mexico, 17–21 September 2003; pp. 3141–3144. [Google Scholar]
- Marques, P.; Marques de Sa, J.P.; Bernardes, J.; Sousa, P. Remote foetal heart rate acquisition and analysis. In Proceedings of the Computers in Cardiology, Cambridge, MA, USA, 24–27 September 2000; pp. 573–574. [Google Scholar]
- Colley, N.; Talbert, D.G.; Southall, D.P. Biophysical profile in the fetus from a phonographic sensor. Eur. J. Obstet. Gynecol. Reprod. Biol. 1986, 23, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Talbert, D.G.; Davies, W.L.; Johnson, F.; Abraham, N.; Colley, N.; Southall, D.P. Wide Bandwidlt Fetal Phonography Using a Sensor Matched to the Compliance of the Mother’s Abdominal Wall. IEEE Trans. Biomed. Eng. 1986, BME-33, 175–181. [Google Scholar] [CrossRef]
- Colley, N.; Abraham, N.G.; Fayers, P.; Talbert, D.G.; Davies, W.L.; Southall, D.P. The Fetal Phonogram: A Measure of Fetal Activity. Lancet 1986, 327, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Bassil, H.E.; Dripps, J.H. Real time processing and analysis of fetal phonocardiographic signals. Clin. Phys. Physiol. Meas. 1989, 10, 67–74. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.; Dripps, J.H.; Grant, P. Processing and analysis of foetal phonocardiograms. In Proceedings of the Images of the Twenty-First Century. Proceedings of the Annual International Engineering in Medicine and Biology Society, Seattle, WA, USA, 9–12 November 1989; pp. 61–62.
- Yao, Y.; Ning, Z.; Zhang, Q.; Zhu, T. Paris: Passive and continuous fetal heart monitoring system. Smart Health 2020, 17, 100087. [Google Scholar] [CrossRef]
- Khandoker, A.; Ibrahim, E.; Oshio, S.; Kimura, Y. Validation of beat by beat fetal heart signals acquired from four-channel fetal phonocardiogram with fetal electrocardiogram in healthy late pregnancy. Sci. Rep. 2018, 8, 13635. [Google Scholar] [CrossRef]
- Aravindan, M.; Alex, J.S.R.; Shwetha, S.; Sri, S.V.; Fathima, A.A.; Kokila, P.R.P. Fetal Health Assessment Through Remote Fetal Phonocardiography and Electrohysterography: Wearable Wireless Device Using Shakti Processor. IEEE Sens. J. 2023, 23, 16219–16226. [Google Scholar] [CrossRef]
- Tan, B.H.; Moghavvemi, M. Real time analysis of fetal phonocardiography. In Proceedings of the 2000 TENCON Proceedings. Intelligent Systems and Technologies for the New Millennium (Cat. No.00CH37119), Kuala Lumpur, Malaysia, 24–27 September 2000; Volume 2, pp. 135–140. [Google Scholar]
- Moghavvemi, M.; Tan, B.; Tan, S. A non-invasive PC-based measurement of fetal phonocardiography. Sens. Actuators A Phys. 2003, 107, 96–103. [Google Scholar] [CrossRef]
- Zhdanov, D.; Bureev, A.; Kosteley, Y. 24-Hour Fetal/Maternal Monitoring System Based on Phonocardiogram Analysis. MATEC Web Conf. 2018, 155, 01046. [Google Scholar] [CrossRef]
- Zhdanov, D.S.; Bureev, A.S.; Kostelei, Y.V.; Khokhlova, L.A.; Dikman, E.Y. A Mobile Device for Assessing Fetal Status Based on Monitoring Cardiovascular System Parameters. Biomed. Eng. 2018, 52, 87–91. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Kepak, S.; Cubik, J.; Kahankova, R.; Janku, P.; Vasinek, V.; Nazeran, H.; Martinek, R. Noninvasive Fetal Heart Rate Monitoring: Validation of Phonocardiography-Based Fiber-Optic Sensing and Adaptive Filtering Using the NLMS Algorithm. Adv. Electr. Electron. Eng. 2017, 15, 544–552. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Zavadil, J.; Vasinek, V. Fetal heart rrate processing based on adaptive least mean squared algorithm. In Proceedings of the 2017 40th International Conference on Telecommunications and Signal Processing (TSP), Barcelona, Spain, 5–7 July 2017; pp. 415–419. [Google Scholar]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Vašinek, V. Processing of fetal heart rate through non-invasive adaptive system based on recursive least squares algorithm. In Optical Materials and Biomaterials in Security and Defence Systems Technology XIV; Zamboni, R., Kajzar, F., Szep, A.A., Matczyszyn, K., Eds.; SPIE: Bellingham, WA, USA, 2017; p. 30. [Google Scholar]
- Martinek, R.; Nedoma, J.; Fajkus, M.; Kahankova, R.; Konecny, J.; Janku, P.; Kepak, S.; Bilik, P.; Nazeran, H. A Phonocardiographic-Based Fiber-Optic Sensor and Adaptive Filtering System for Noninvasive Continuous Fetal Heart Rate Monitoring. Sensors 2017, 17, 890. [Google Scholar] [CrossRef] [PubMed]
- Martinek, R.; Kahankova, R.; Nedoma, J.; Fajkus, M.; Nazeran, H.; Nowakova, J. Adaptive Signal Processing of Fetal PCG Recorded by Interferometric Sensor. In Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2018; pp. 235–243. [Google Scholar]
- Wang, Z.; Jiang, H. Wireless intelligent sensor system for fetal heart rate tracing through body sound monitoring on a pregnant woman. In Proceedings of the 2013 IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-BIO), Singapore, 9–11 December 2013; pp. 1–3. [Google Scholar]
- Yang, W.; Yang, K.; Jiang, H.; Wang, Z.; Lin, Q.; Jia, W. Fetal heart rate monitoring system with mobile internet. In Proceedings of the 2014 IEEE International Symposium on Circuits and Systems (ISCAS), Melbourne, VIC, Australia, 1–5 June 2014; pp. 443–446. [Google Scholar]
- Mittra, A.K.; Shukla, A.; Zadgaonkar, A.S.; Choudhary, N.K. An Improved Method of Long-Term Fetal Heart Sound Monitoring in High-Risk Pregnancies. IETE J. Res. 2007, 53, 513–521. [Google Scholar] [CrossRef]
- Varady, P. Wavelet-based adaptive denoising of phonocardiographic records. In Proceedings of the 2001 Conference Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; pp. 1846–1849.
- Várady, P.; Wildt, L.; Benyó, Z.; Hein, A. An advanced method in fetal phonocardiography. Comput. Methods Programs Biomed. 2003, 71, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Goovaerts, H.G.; Van Geijn, H.P.; Rompelman, O. An Inductive Sensor For Recording Of Fetal Movements And Sounds. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 31 October–3 November 1991; pp. 1622–1623. [Google Scholar]
- Goovaerts, H.G.; Wilmsen, A.A.; Cortenraad, M.G.G.; Van Geijn, H.P.; Rompelman, O. Recording and processing of fetal movements and sounds obtained with the Inpho inductive transducer. Med. Biol. Eng. Comput. 1991, 29, NS20–NS26. [Google Scholar] [CrossRef] [PubMed]
- Dressler, M.; Moskowitz, S.N. Fetal electrocardiography and stethography. Am. J. Obstet. Gynecol. 1941, 41, 775–791. [Google Scholar] [CrossRef]
- Bartolucci, L.; Webb, G.A. A multiple-channel fetal heart tone monitoring system for routine clinical use. Am. J. Obstet. Gynecol. 1970, 107, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.; Albazzaz, S. Measurement of Pulmonary Capillary Pressure During Ritodrine Tocolysis in Twin Pregnancies: A New Noninvasive Technique. Am. J. Perinatol. 1993, 10, 351–353. [Google Scholar] [CrossRef]
- Lakshmi, G.S.; Bhaskaran, A.; Bharadwaj, S.U.; Arora, M. Effect of Contact Force on Foetal Heart Sound Recordings. In Proceedings of the 2020 IEEE International Conference on Electronics, Computing and Communication Technologies (CONECCT), Bangalore, India, 2–4 July 2020; pp. 1–5. [Google Scholar]
- Samieinasab, M.; Sameni, R. Fetal phonocardiogram extraction using single channel blind source separation. In Proceedings of the 2015 23rd Iranian Conference on Electrical Engineering, Tehran, Iran, 10–14 May 2015; pp. 78–83. [Google Scholar]
- Jimenez, A.; Ortiz, M.R.; Pena, M.A.; Charleston, S.; Gonzalez, R.; Aljama, A.T.; Carrasco, S. Performance of a method to generate fetal cardiotachograms using fetal phonocardiography. In Proceedings of the Computers in Cardiology, Rotterdam, The Netherlands, 23–26 September 2001; pp. 453–456. [Google Scholar]
- Bhaskaran, A.; Kumar, J.S.; George, S.; Arora, M. Heart rate estimation and validation algorithm for fetal phonocardiography. Physiol. Meas. 2022, 43, 075008. [Google Scholar] [CrossRef]
- Gobillot, S.; Fontecave-Jallon, J.; Equy, V.; Rivet, B.; Gumery, P.Y.; Hoffmann, P. Non-invasive fetal monitoring using electrocardiography and phonocardiography: A preliminary study. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 455–459. [Google Scholar] [CrossRef]
- Dia, N.; Fontecave-Jallon, J.; Gumery, P.-Y.; Rivet, B. Fetal heart rate estimation from a single phonocardiogram signal using non-negative matrix factorization. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5983–5986. [Google Scholar]
- Faisant, M.; Fontecave-Jallon, J.; Genoux, B.; Rivet, B.; Dia, N.; Resendiz, M.; Riethmuller, D.; Equy, V.; Hoffmann, P. Non-invasive fetal monitoring: Fetal Heart Rate multimodal estimation from abdominal electrocardiography and phonocardiography. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102421. [Google Scholar] [CrossRef] [PubMed]
- Souriau, R.; Fontecave-Jallon, J.; Rivet, B. Fetal heart rate monitoring by fusion of estimations from two modalities: A modified Viterbi’s algorithm. Biomed. Signal Process. Control 2023, 80, 104405. [Google Scholar] [CrossRef]
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. [Google Scholar] [CrossRef] [PubMed]















| Database | Search Parameters | Query | Accessed on |
|---|---|---|---|
| Scopus | Article title, abstract, keywords | (“Fetal” OR “Pregnancy” OR “Fetus” OR “Prenatal” OR “Antenatal” OR “Foetal” OR “Foetus”) AND (“Phonocardiography” OR “Heart Sound” OR “FPCG” OR “PCG” OR “Heart Murmur” OR “Acoustic cardiography” OR “Auscultation”) AND (“Hardware” OR “Device” OR “System” OR “Recording” OR “Acquisition” OR “Microphone”) | 2 November 2023 |
| PubMed | All fields | 2 November 2023 | |
| Web of Science | Title, abstract, Keyword Plus ® | 2 November 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, N.; Sbrollini, A.; Morettini, M.; Rosati, S.; Balestra, G.; Gambi, E.; Knaflitz, M.; Burattini, L. Acquisition Devices for Fetal Phonocardiography: A Scoping Review. Bioengineering 2024, 11, 367. https://doi.org/10.3390/bioengineering11040367
Giordano N, Sbrollini A, Morettini M, Rosati S, Balestra G, Gambi E, Knaflitz M, Burattini L. Acquisition Devices for Fetal Phonocardiography: A Scoping Review. Bioengineering. 2024; 11(4):367. https://doi.org/10.3390/bioengineering11040367
Chicago/Turabian StyleGiordano, Noemi, Agnese Sbrollini, Micaela Morettini, Samanta Rosati, Gabriella Balestra, Ennio Gambi, Marco Knaflitz, and Laura Burattini. 2024. "Acquisition Devices for Fetal Phonocardiography: A Scoping Review" Bioengineering 11, no. 4: 367. https://doi.org/10.3390/bioengineering11040367
APA StyleGiordano, N., Sbrollini, A., Morettini, M., Rosati, S., Balestra, G., Gambi, E., Knaflitz, M., & Burattini, L. (2024). Acquisition Devices for Fetal Phonocardiography: A Scoping Review. Bioengineering, 11(4), 367. https://doi.org/10.3390/bioengineering11040367