Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review
Abstract
:1. Introduction
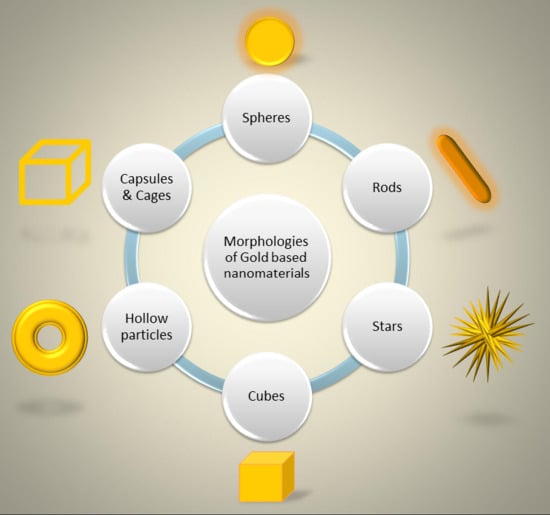
2. Engineered Gold-Based Nanomaterials
2.1. Gold Nanospheres
2.2. Gold Nanorods
2.3. Gold Nanostars
2.4. Gold Nanocubes
2.5. Gold Hollow Nanoparticles and Nanocapsules
3. Key Properties for Biomedicine
4. Prospective Outlooks and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. Engl. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhu, Y.; Liu, B.; Quan, G.; Cui, L. Colorimetric Determination of Hypochlorite Based on the Oxidative Leaching of Gold Nanorods. Materials 2018, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Pantalei, S.; Zampetti, E.; Macagnano, A.; Bearzotti, A.; Venditti, I.; Russo, M.V. Enhanced sensory properties of a multichannel quartz crystal microbalance coated with polymeric nanobeads. Sensors 2007, 7, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.F.; Pang, J.; You, Q.N.; Liu, T.; Chu, Z.Y.; Jin, W.Q. Simultaneous biosensing of catechol and hydroquinone via a truncated cube-shaped Au/PBA nanocomposite. Biosens. Bioelectron. 2019, 124, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; Fratoddi, I.; Bearzotti, A. Self-assembled copolymeric nanoparticles as chemically interactive materials for humidity sensors. Nanotechnology 2010, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef]
- D’Amato, R.; Venditti, I.; Russo, M.V.; Falconieri, M. Growth Control and Long range Self-assembly of Polymethylmethacrylate Nanospheres. J. Appl. Polym. Sci. 2006, 102, 4493–4499. [Google Scholar] [CrossRef]
- Luo, C.D.; Wang, Y.Y.; Li, X.M.; Jiang, X.Q.; Gao, P.P.; Sun, K.; Zhou, J.H.; Zhang, Z.G.; Jiang, Q. An Optical Sensor with Polyaniline-Gold Hybrid Nanostructures for Monitoring pH in Saliva. Nanomaterials 2017, 7, 11. [Google Scholar] [CrossRef]
- Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferrato, R. Sensitivity to Heavy-Metal Ions of Unfolded Fullerene Quantum Dots. Sensors 2017, 17, 2614. [Google Scholar] [CrossRef]
- Chen, Z.; Choi, C.K.K.; Wang, Q. Origin of the Plasmonic Chirality of Gold Nanorod Trimers Templated by DNA Origami. ACS Appl. Mater. Interfaces 2018, 10, 26835–26840. [Google Scholar] [CrossRef]
- Matassa, R.; Familiari, G.; Battaglione, E.; Sibilia, C.; Leahu, G.; Belardini, A.; Venditti, I.; Fontana, L.; Fratoddi, I. Electron microscopy reveals a soluble hybrid network of individual nanocrystals self-anchored by bifunctional thiol fluorescent bridges. Nanoscale 2016, 8, 18161–18169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Caldarola, M.; Lu, X.; Pradhan, B.; Orrit, M. Single-molecule fluorescence enhancement of a near-infrared dye by gold nanorods using DNA transient binding. Phys. Chem. Chem. Phys. 2018, 20, 20468–20475. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; D’Amato, R.; Russo, M.V.; Falconieri, M. Synthesis of conjugated polymeric nanobeads for photonic bandgap materials. Sens. Actuators B-Chem. 2007, 126, 35–40. [Google Scholar] [CrossRef]
- Vestler, D.; Shishkin, I.; Gurvitz, E.A.; Nasir, M.E.; Ben-Moshe, A.; Slobozhanyuk, A.P.; Krasavin, A.V.; Levi-Belenkova, T.; Shalin, A.S.; Ginzburg, P.; et al. Circular dichroism enhancement in plasmonic nanorod metamaterials. Opt. Express 2018, 26, 17841–17848. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.; Wang, R.; He, Y.Y.; Kirby, R.M.; Dal Negro, L. Optimization of Large-Scale Vogel Spiral Arrays of Plasmonic Nanoparticles. Plasmonics 2019, 14, 253–261. [Google Scholar] [CrossRef]
- Mousli, F.; Chaouchi, A.; Hocine, S.; Lamouri, A.; Vilar, M.R.; Kadri, A.; Chehimi, M.M. Diazonium-modified TiO2/polyaniline core/shell nanoparticles. Structural characterization, interfacial aspects and photocatalytic performances. Appl. Surf. Sci. 2019, 465, 1078–1095. [Google Scholar] [CrossRef]
- Naponiello, G.; Venditti, I.; Zardetto, V.; Saccone, D.; Di Carlo, A.; Fratoddi, I.; Barolo, C.; Dini, D. Photoelectrochemical characterization of squaraine-sensitized nickel oxide cathodes deposited via screen-printing for p-type dye-sensitized solar cells. Appl. Surf. Sci. 2015, 356, 911–920. [Google Scholar] [CrossRef]
- Chang, S.; Li, Q.; Xiao, X.; Wong, K.Y.; Chen, T. Enhancement of low energy sunlight harvesting in dye-sensitized solar cells using plasmonic gold nanorods. Energ. Environ. Sci. 2012, 5, 9444–9448. [Google Scholar] [CrossRef]
- Ali, A.K.; Erten-Ela, S.; Hassoon, K.I.; Ela, C. Plasmonic enhancement as selective scattering of gold nanoparticles based dye sensitized solar cells. Thin Solid Films 2019, 671, 127–132. [Google Scholar] [CrossRef]
- Bonomo, M.; Naponiello, G.; Venditti, I.; Zardetto, V.; Di Carlo, A.; Dini, D. Electrochemical and Photoelectrochemical Properties of Screen-Printed Nickel Oxide Thin Films Obtained from Precursor Pastes with Different Compositions. J. Electrochem. Soc. 2017, 164, H137–H147. [Google Scholar] [CrossRef]
- Chong, S.; Yang, T.C.K. Parametric Studies of Titania-Supported Gold-Catalyzed Oxidation of Carbon Monoxide. Materials 2017, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Li, Z.C.; Deng, Y.J.; Zhao, Z.H.; Li, X.W.; Xia, Y.Z. Facile Synthesis of Gold Nanoparticles with Alginate and Its Catalytic Activity for Reduction of 4-Nitrophenol and H2O2 Detection. Materials 2017, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Seo, M.G.; Choi, C.; Kim, J.S.; Jung, E.; Hang, G.H.; Lee, J.C.; Han, S.S.; Ahn, J.P.; Jung, Y.; et al. Studies on Catalytic Activity of Hydrogen Peroxide Generation according to Au Shell Thickness of Pd/Au Nanocubes. ACS Appl. Mater. Interfaces 2018, 10, 38109–38116. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, F.; Carlini, L.; Ugolini, A.; Visaggio, D.; Visca, P.; Fratoddi, I.; Venditti, I.; Meneghini, C.; Simonelli, L.; Marini, C.; et al. Synthesis and Structural Characterization of Silver Nanoparticles Stabilized with 3-Mercapto-1-Propansulfonate and 1-Thioglucose Mixed Thiols for Antibacterial Applications. Materials 2016, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Fasolato, C.; Giantulli, S.; Silvestri, I.; Mazzarda, F.; Toumia, Y.; Ripanti, F.; Mura, F.; Luongo, F.; Costantini, F.; Bordi, F.; et al. Folate-based single cell screening using surface enhanced Raman microimaging. Nanoscale 2016, 8, 17304–17313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Ku, M.; Lee, H.; Yoon, N.; Yang, J.; Bong, K.W. Implantable Photothermal Agents based on Gold Nanorods-Encapsulated Microcube. Sci. Rep. 2018, 8, 13683. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2017. [Google Scholar] [CrossRef]
- Bassi, B.; Dacarro, G.; Galinetto, P.; Giulotto, E.; Marchesi, N.; Pallavicini, P.; Pascale, A.; Perversi, S.; Taglietti, A. Tailored coating of gold nanostars: Rational approach to prototype of theranostic device based on SERS and photothermal effects at ultralow irradiance. Nanotechnology 2018, 8, 235301. [Google Scholar] [CrossRef]
- Placido, T.; Tognaccini, L.; Howes, B.D.; Montrone, A.; Laquintana, V.; Comparelli, R.; Curri, M.L.; Smulevich, G.; Agostiano, A. Surface Engineering of Gold Nanorods for Cytochrome. ACS Omega 2018, 3, 4959–4967. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zheng, M.X.; Wu, J.C.; Li, C.; Shen, D.Q.; Yang, D.; Li, L.; Ge, M.F.; Chang, Z.M.; Dong, W.F. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, T.; Choi, J.W. Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells. Nanomaterials 2016, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venditti, I. Gold Nanoparticles in Photonic Crystals Applications: A Review. Materials 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Bearzotti, A.; Papa, P.; Macagnano, A.; Zampetti, E.; Venditti, I.; Fioravanti, R.; Fontana, L.; Matassa, R.; Familiari, G.; Fratoddi, I. Environmental Hg vapours adsorption and detection by using functionalized gold nanoparticles network. J. Environ. Chem. Eng. 2018, 6, 4706–4713. [Google Scholar] [CrossRef]
- Pedrosa, P.; Vinhas, R.; Fernandes, A.; Baptista, P.V. Gold Nanotheranostics: Proof-of-Concept or Clinical Tool? Nanomaterials 2015, 5, 1853–1879. [Google Scholar] [CrossRef] [Green Version]
- Gharpure, K.M.; Wu, S.Y.; Li, C.; Lopez-Berestein, G.; Sood, A.K. Nanotechnology: Future of Oncotherapy. Clin. Cancer Res. 2015, 21, 3121–3130. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Stender, A.S.; Sun, W.; Fang, N. Optical imaging of non-fluorescent nanoparticle probes in live cells. Analyst 2010, 135, 215–221. [Google Scholar] [CrossRef]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Durr, N.J.; Larson, T.; Smith, D.K.; Korgel, B.A.; Sokolov, K.; Ben-Yakar, A. Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods. Nano Lett. 2007, 7, 941–945. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Zhang, Q.; Cobley, C.M.; Gidding, M.; Schwartz, A.G.; Chen, J.; Xia, Y. Quantifying the cellular uptake of antibody-conjugated Au nanocages by two-photon microscopy and inductively coupled plasma mass spectrometry. ACS Nano 2010, 4, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Jing, Y.; Xu, Y.H.; Bhattacharya, R.; Mukhopadhyay, D.; Glockner, J.F.; Wang, J.P.; Mukherjee, P. A core-shell nanomaterial with endogenous therapeutic and diagnostic functions. Cancer Nanotechnol. 2010, 1, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutter, E.; Boridy, S.; Labrecque, S.; Lalancette-Hébert, M.; Kriz, J.; Winnik, F.M.; Maysinger, D. Microglial response to gold nanoparticles. ACS Nano 2010, 4, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. One low-dose exposure of gold nanoparticles induces long-term changes in human cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13318–13323. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Donati, S.; Fontana, L.; Porcaro, F.; Battocchio, C.; Proietti, E.; Venditti, I.; Bracci, L.; Fratoddi, I. Negatively charged gold nanoparticles as a dexamethasone carrier: Stability in biological media and bioactivity assessment in vitro. RSC Adv. 2016, 6, 99016–99022. [Google Scholar] [CrossRef]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicol. Res. 2015, 4, 796–800. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Mironava, T.; Hadjiargyrou, M.; Simon, M.; Jurukovski, V.; Rafailovich, M.H. Gold nanoparticles cellular toxicity and recovery: Effect of size, concentration and exposure time. Nanotoxicology 2010, 4, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Carlini, L.; Fasolato, C.; Postorino, P.; Fratoddi, I.; Venditti, I.; Testa, G.; Battocchio, C. Comparison between silver and gold nanoparticles stabilized with negatively charged hydrophilic thiols: SR-XPS and SERS as probes for structural differences and similarities. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 532, 183–188. [Google Scholar] [CrossRef]
- Fratoddi, I.; Matassa, R.; Fontana, L.; Venditti, I.; Familiari, G.; Battocchio, C.; Magnano, E.; Nappini, S.; Leahu, G.; Belardini, A.; et al. Electronic Properties of a Functionalized Noble Metal Nanoparticles Covalent Network. J. Phys. Chem. C 2017, 121, 18110–18119. [Google Scholar] [CrossRef]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Porcaro, F.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic Metal Nanoparticles Functionalized by 2-Diethylaminoethanethiol: A Close Look at the Metal-Ligand Interaction and Interface Chemical Structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cartoni, A.; Venditti, I.; Catone, D.; O’Keeffe, P.; Paladini, A.; Toschi, F.; Turchini, S.; Sciubba, F.; Testa, G.; et al. Gold nanoparticles functionalized by rhodamine B isothiocyanate: A new tool to control plasmonic effects. J. Colloid Interface Sci. 2018, 513, 10–19. [Google Scholar] [CrossRef]
- Bessar, H.; Venditti, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; Federici, M.; et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surf. B 2016, 141, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Porcaro, F.; Battocchio, C.; Antoccia, A.; Fratoddi, I.; Venditti, I.; Fracassi, A.; Luisetto, I.; Russo, M.V.; Polzonetti, G. Synthesis of functionalized gold nanoparticles capped with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols and “in vitro” bioresponse. Colloids Surf. B 2016, 142, 408–416. [Google Scholar] [CrossRef]
- Venditti, I.; Hassanein, T.F.; Fratoddi, I.; Fontana, L.; Battocchio, C.; Rinaldi, F.; Carafa, M.; Marianecci, C.; Diociaiuti, M.; Agostinelli, E.; et al. Bioconjugation of gold-polymer core-shell nanoparticles with bovine serum amine oxidase for biomedical applications. Colloids Surf. B 2015, 134, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Rodzik-Czalka, L.; Lewandowska-Lancucka, J.; Gatta, V.; Venditti, I.; Fratoddi, I.; Szuwarzynski, M.; Romek, M.; Nowakowska, M. Nucleobases functionalized quantum dots and gold nanoparticles bioconjugates as a fluorescence resonance energy transfer (FRET) system - Synthesis, characterization and potential applications. J. Colloid Interface Sci. 2018, 514, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Kim, M.J. Influence of the photothermal effect of a gold nanorod cluster on biofilm disinfection. Nanotechnology 2013, 24, 195104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ramasamy, M.; Yi, D.K. Antibacterial Activity of Ordered Gold Nanorod Arrays. ACS Appl. Mater. Interfaces 2014, 6, 15078–15085. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Bassi, B.; Chirico, G.; Collini, M.; Dacarro, G.; Fratini, E.; Grisoli, P.; Patrini, M.; Sironi, L.; Taglietti, A.; et al. Modular approach for bimodal antibacterial surfaces combining photo-switchable activity and sustained biocidal release. Sci. Rep. 2017, 7, 5259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, K.C.L.; Sileika, T.S.; Yi, J.; Zhang, R.; Rivera, J.G.; Messersmith, P.B. Bacterial killing by light-triggered release of silver from biomimetic metal nanorods. Small 2014, 10, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, L.; Kora, A.J.; Arunachalam, J. Highly stable, protein capped gold nanoparticles as effective drug delivery vehicles for amino-glycosidic antibiotics. Mater. Sci. Eng. C 2012, 32, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Roshmi, T.; Soumya, K.R.; Jyothis, M.; Radhakrishnan, E.K. Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 2015, 48, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef]
- Wang, X.L.; Wei, C.C.; Liu, M.K.; Yang, T.; Zhou, W.M.; Liu, Y.; Hong, K.; Wang, S.H.; Xin, H.B.; Ding, X.W. Near-Infrared Triggered Release of uPA from Nanospheres for Localized Hyperthermia-Enhanced Thrombolysis. Adv. Funct. Mater. 2017, 27, 8. [Google Scholar] [CrossRef]
- Yohan, D.; Yang, C.; Lu, X.F.; Chithrani, D.B. Size dependent gold nanoparticle interaction at nano-micro interface using both monolayer and multilayer (tissue-like) cell models. In Colloidal Nanoparticles for Biomedical Applications Xi; Parak, W.J., Osinski, M., Liang, X.J., Eds.; SPIE-Int Soc Optical Engineering: Bellingham, WA, USA, 2016; Volume 9722. [Google Scholar]
- Zhou, B.; Song, J.; Wang, M.; Wang, X.; Wang, J.; Howard, E.W.; Zhou, F.; Qu, J.; Chen, W.R. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale 2018, 46. [Google Scholar] [CrossRef] [PubMed]
- Karabel Ocal, S.; Patarroyo, J.; Kiremitler, N.B.; Pekdemir, S.; Puntes, V.F.; Onses, M.S. Plasmonic assemblies of gold nanorods on nanoscale patterns of poly(ethylene glycol): Application in surface-enhanced Raman spectroscopy. J. Colloid Interface Sci. 2018, 532, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Lee, Y.; Nam, J.M. Precisely Shaped, Uniformly Formed Gold Nanocubes with Ultrahigh Reproducibility in Single-Particle Scattering and Surface-Enhanced Raman Scattering. Nano Lett. 2018, 18, 6475–6482. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, J.K.; Li, J.J.; Zhao, J.W. Local dielectric environment-dependent plasmonic optical sensitivity of gold nanocage: From nanobox to nanoframe. Appl. Phys. A-Mater. Sci. Process. 2019, 125, 11. [Google Scholar] [CrossRef]
- Xu, X.Y.; Chong, Y.; Liu, X.Y.; Fu, H.; Yu, C.G.; Huang, J.; Zhang, Z.J. Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta Biomater. 2019, 84, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Xin, H.L.; Xia, W.W.; Liu, M.Z.; Zhang, Y.G.; Cai, W.P.; Gang, O. Tailoring Surface Opening of Hollow Nanocubes and Their Application as Nanocargo Carriers. ACS Central Sci. 2018, 4, 1742–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Li, S.Y.; Gu, Y.J.; Xiong, H.H.; Wong, W.T.; Sun, L. Multispectral Photoacoustic Imaging of Tumor Protease Activity with a Gold Nanocage-Based Activatable Probe. Mol. Imaging Biol. 2018, 20, 919–929. [Google Scholar] [CrossRef]
- Sun, Y.W.; Wang, L.H.; Meng, D.L.; Che, X. A green and facile preparation approach, licochalcone A capped on hollow gold nanoparticles, for improving the solubility and dissolution of anticancer natural product. Oncotarget 2017, 8, 105673–105681. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.M.; Wang, H.J.; Du, H. Colloidal gold nanorings for improved photodynamic therapy through field-enhanced generation of reactive oxygen species. In Colloidal Nanocrystals for Biomedical Applications Viii; Parak, W.J., Osinski, M., Yamamoto, K., Eds.; SPIE-Int Soc Optical Engineering: Bellingham, WA, USA, 2013; Volume 8595. [Google Scholar]
- Pylaev, T.; Vanzha, E.; Avdeeva, E.; Khlebtsov, B.; Khlebtsov, N. A novel cell transfection platform based on laser optoporation mediated by Au nanostar layers. J. Biophotonics 2019, 12, 12. [Google Scholar] [CrossRef]
- Duong, H.D.; Vo-Dinh, T.; Rhee, J.I. Synthesis and functionalization of gold nanostars for singlet oxygen production. J. Ind. Eng. Chem. 2019, 69, 233–240. [Google Scholar] [CrossRef]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Du, H.; Ren, L.; Wang, H. Colloidal plasmonic gold nanoparticles and gold nanorings: Shape-dependent generation of singlet oxygen and their performance in enhanced photodynamic cancer therapy. Int. J. Nanomed. 2018, 13, 2065–2078. [Google Scholar] [CrossRef] [PubMed]
- Bassi, B.; Taglietti, A.; Galinetto, P.; Marchesi, N.; Pascale, A.; Cabrini, E.; Pallavicini, P.; Dacarro, G. Tunable coating of gold nanostars: Tailoring robust SERS labels for cell imaging. Nanotechnology 2016, 27, 265302. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, D.; Seo, Y.; Son, J.; Jo, Y.; Lee, K.; Park, C.; Choi, J. Engineered nanomaterials for their applications in theragnostics. J. Ind. Eng. Chem. 2018, 66, 20–28. [Google Scholar] [CrossRef]
- Dam, D.H.; Culver, K.S.; Odom, T.W. Grafting aptamers onto gold nanostars increases in vitro efficacy in a wide range of cancer cell types. Mol. Pharm. 2014, 11, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Teng, Z.; Tian, W.; Luo, S.; Kong, X.; Su, X.; Tang, Y.; Wang, S.; Lu, G. pH-Dependent Transmembrane Activity of Peptide-Functionalized Gold Nanostars for Computed Tomography/Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2114–2122. [Google Scholar] [CrossRef]
- Casu, A.; Cabrini, E.; Donà, A.; Falqui, A.; Diaz-Fernandez, Y.; Milanese, C.; Taglietti, A.; Pallavicini, P. Controlled synthesis of gold nanostars by using a zwitterionic surfactant. Chemistry 2012, 18, 9381–9390. [Google Scholar] [CrossRef]
- Masud, M.K.; Yadav, S.; Isam, M.N.; Nguyen, N.T.; Salomon, C.; Kline, R.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; Hossain, M.S.A.; et al. Gold-Loaded Nanoporous Ferric Oxide Nanocubes with Peroxidase-Mimicking Activity for Electrocatalytic and Colorimetric Detection of Autoantibody. Anal. Chem. 2017, 89, 11005–11013. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Ming, T.; Wang, X.; Wang, P.; Wang, J.; Chen, J. High-photoluminescence-yield gold nanocubes: For cell imaging and photothermal therapy. ACS Nano 2010, 4, 113–120. [Google Scholar] [CrossRef]
- Thiele, M.; Soh, J.Z.E.; Knauer, A.; Malsch, D.; Stranik, O.; Muller, R.; Csaki, A.; Henkel, T.; Kohler, J.M.; Fritzsche, W. Gold nanocubes—Direct comparison of synthesis approaches reveals the need for a microfluidic synthesis setup for a high reproducibility. Chem. Eng. J. 2016, 288, 432–440. [Google Scholar] [CrossRef]
- Yoon, S.; Rossi, J.J. Targeted Molecular Imaging Using Aptamers in Cancer. Pharmaceuticals 2018, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Liu, F.; Yang, Y.T.; Wu, Y.; Tian, W.Q. Strategies on Nanodiagnostics and Nanotherapies of the Three Common Cancers. Nanomaterials 2018, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Kuo, B.H.; Hsia, C.F.; Chen, T.N.; Huang, M.H. Systematic Shape Evolution of Gold Nanocrystals Achieved through Adjustment in the Amount of HAuCl4 Solution Used. J. Phys. Chem. C 2018, 122, 25118–25126. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, N.; Kim, J.H.; Il Park, Y.; Piao, Y. Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications. Nanomaterials 2018, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Wang, L.L.; Ringe, E. Small morphology variations effects on plasmonic nanoparticle dimer hotspots. J. Mater. Chem. C 2018, 6, 9607–9614. [Google Scholar] [CrossRef]
- Dayem, A.A.; Bin Lee, S.; Cho, S.G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 32. [Google Scholar]
- D’Acunto, M. Detection of Intracellular Gold Nanoparticles: An Overview. Materials 2018, 11, 882. [Google Scholar] [CrossRef]
- Cancino-Bernardi, J.; Marangoni, V.S.; Besson, J.C.F.; Cancino, M.E.C.; Natali, M.R.M.; Zucolotto, V. Gold-based nanospheres and nanorods particles used as theranostic agents: An in vitro and in vivo toxicology studies. Chemosphere 2018, 213, 41–52. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, W.; Zhang, J.; Li, H.; Han, H.; Zhai, T. Design of Gold Hollow Nanorods with Controllable Aspect Ratio for Multimodal Imaging and Combined Chemo-Photothermal Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2018, 10, 43. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; McDonagh, B.H.; Singh, G.; Raghunathan, K.; Sandvig, A.; Sandvig, I.; Andreassen, J.P.; Glomm, W.R. Growing gold nanostructures for shape-selective cellular uptake. Nanoscale Res. Lett. 2018, 13, 254. [Google Scholar] [CrossRef]
- Turcu, I.; Zarafu, I.; Popa, M.; Chifiriuc, M.C.; Bleotu, C.; Culita, D.; Ghica, C.; Ionita, P. Lipoic Acid Gold Nanoparticles Functionalized with Organic Compounds as Bioactive Materials. Nanomaterials 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Yu, J.; Go, M.R.; Kim, H.J.; Hwang, Y.G.; Choi, S.J. Oral Toxicity and Intestinal Transport Mechanism of Colloidal Gold Nanoparticle-Treated Red Ginseng. Nanomaterials 2016, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Chen, S.M.; Cheng, F.; Wang, H.Q.; Peng, W. Surface Plasmon Resonance Biosensor Based on Smart Phone Platforms. Sci. Rep. 2015, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Hong, S.; Choi, Y.; Park, J.H.; Nam, Y. Photothermal Inhibition of Neural Activity with Near-Infrared-Sensitive Nanotransducers. ACS Nano 2014, 8, 8040–8049. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Okon, S.L. Nanomaterial-Based Electrochemical Immunosensors for Clinically Significant Biomarkers. Materials 2014, 7, 4669–4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.P.; Wu, S.; Chou, K.C.; Signorell, R. Investigation of Sub-100 nm Gold Nanoparticles for Laser-Induced Thermotherapy of Cancer. Nanomaterials 2013, 3, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Hayden, S.C.; Austin, L.A.; Near, R.D.; Ozturk, R.; El-Sayed, M.A. Plasmonic enhancement of photodynamic cancer therapy. J. Photochem. Photobiol. A-Chem. 2013, 269, 34–41. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer-Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Lin, Y.L.; Jen, J.C.; Hsu, S.H.; Chiu, I.M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70, 9–18. [Google Scholar] [CrossRef]
- Jain, K.K. Applications of nanobiotechnology in clinical diagnostics. Clin. Chem. 2007, 53, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.; Mansour, M.M.H.; Kazmierczak, S.C. Nanodiagnostics: A new frontier for clinical laboratory medicine. Clin. Chem. 2006, 52, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Diagaradjane, P.; Shetty, A.; Wang, J.C.; Elliott, A.M.; Schwartz, J.; Shentu, S.; Park, H.C.; Deorukhkar, A.; Stafford, R.J.; Cho, S.H.; et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: Characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett. 2008, 8, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Lapotko, D.O.; Lukianova-Hleb, E.Y.; Oraevsky, A.A. Clusterization of nanoparticles during their interaction with living cells. Nanomedicine 2007, 2, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.; Ford, M.J.; Cortie, M.B. Optimization of plasmonic heating by gold nanospheres and nanoshells. J. Phys. Chem. B 2006, 110, 10701–10707. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Gormley, A.J.; Greish, K.; Ray, A.; Robinson, R.; Gustafson, J.A.; Ghandehari, H. Gold nanorod mediated plasmonic photothermal therapy: A tool to enhance macromolecular delivery. Int. J. Pharm. 2011, 415, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef]
- Au, L.; Zheng, D.; Zhou, F.; Li, Z.Y.; Li, X.; Xia, Y. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008, 2, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence enhancement by Au nanostructures: Nanoshells and nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Orozco, C.; Liu, J.G.; Knight, M.W.; Wang, Y.; Day, J.K.; Nordlander, P.; Halas, N.J. Fluorescence enhancement of molecules inside a gold nanomatryoshka. Nano Lett. 2014, 14, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Leuvering, J.H.; Thal, P.J.; van der Waart, M.; Schuurs, A.H. Sol particle immunoassay (SPIA). J. Immunoass. 1980, 1, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.F.; Lau, P.M.; Kong, S.K.; Ho, H.P. An Assay Using Localized Surface Plasmon Resonance and Gold Nanorods Functionalized with Aptamers to Sense the Cytochrome-c Released from Apoptotic Cancer Cells for Anti-Cancer Drug Effect Determination. Micromachines 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Bogatyrev, V.A.; Khlebtsov, B.N.; Khlebtsov, N.G. A protein assay based on colloidal gold conjugates with trypsin. Anal. Biochem. 2005, 341, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Bogatyrev, V.; Dykman, L.; Khlebtsov, B.; Staroverov, S.; Shirokov, A.; Matora, L.; Khanadeev, V.; Pylaev, T.; Tsyganova, N.; et al. Analytical and theranostic applications of gold nanoparticles and multifunctional nanocomposites. Theranostics 2013, 3, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa; Vidya, S.M.; Mutalik, S.; Udaya Bhat, K.; Huilgol, P.; Avadhani, K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016, 153, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Liu, G.; Zhang, G.; Hu, J.; Liu, S. Engineering Cross-Linkable Plasmonic Vesicles for Synergistic Chemo-Photothermal Therapy Using Orthogonal Light Irradiation. Macromolecules 2018, 51, 8530–8538. [Google Scholar] [CrossRef]
- Fratoddi, I.; Benassi, L.; Vaschieri, C.; Venditti, I.; Bessar, H.; Mai, S.; Azzoni, P.; Magnoni, C.; Costanzo, A.; Casagrande, V.; et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine 2019, 17, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.Y.R.; Proenza, Y.G.; da Luz, L.L.; de Sousa Araújo, S.; Filho, M.A.G.; Junior, S.A.; Soares, T.A.; Longo, R.L. A thermo-responsive adsorbent-heater-thermometer nanomaterial for controlled drug release: (ZIF-8,Eux Tby)@AuNP core-shell. Mater. Sci. Eng. C 2019, 102, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Zhao, J.; Cao, S.; Brodie, P.J.; Tamarkin, L.; Huhta, M.; Myer, L.D.; Friedman, J.; Kingston, D.G.I. Synthesis and Evaluation of Paclitaxel-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Bioconjug. Chem. 2016, 16, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Porchia, M.; Tisato, F.; Bondino, F.; Magnano, E.; Pellei, M.; Santini, C. Highly hydrophilic gold nanoparticles as carrier for anticancer copper(I) complexes: Loading and release studies for biomedical applications. Nanomaterials 2019, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.R.A.; Lataliza, A.A.B.; Raposo, N.R.B.; Costa, L.A.S.; Sant’Ana, A.C. Insights on the transport of tamoxifen by gold nanoparticles for MCF-7 breast cancer cells based on SERS spectroscopy. Colloids Surf. B 2018, 170, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric in Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Rathinaraj, P.; Al-Jumaily, A.M.; Huh, D.S. Internalization: Acute apoptosis of breast cancer cells using herceptin-immobilized gold nanoparticles. Breast Cancer 2015, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kluenker, M.; Mondeshki, M.; Nawaz Tahir, M.; Tremel, W. Monitoring Thiol-Ligand Exchange on Au Nanoparticle Surfaces. Langmuir 2018, 34, 1700–1710. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Parry, J.S. Strong resistance of citrate anions on metal nanoparticles to desorption under thiol functionalization. ACS Nano 2015, 9, 1665–1682. [Google Scholar] [CrossRef] [PubMed]
- Giovannozzi, A.M.; Rolle, F.; Sega, M.; Abete, M.C.; Marchis, D.; Rossi, A.M. Rapid and sensitive detection of melamine in milk with gold nanoparticles by Surface Enhanced Raman Scattering. Food Chem. 2014, 159, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Bassetti, M.; Battocchio, C.; Venditti, I.; Fratoddi, I. Synthesis of gold and silver nanoparticles functionalized with organic dithiols. Colloid Surf. A 2017, 532, 282–289. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Polzonetti, G.; Cametti, C.; Russo, M.V. Core shell hybrids based on noble metal nanoparticles and conjugated polymers: Synthesis and characterization. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, C.; Zhang, Z.; Jiang, L.; Li, J. A novel nanogold multilayer constructed by Langmuir-Blodgett and self-assembly techniques. J. Colloid Interface Sci. 2005, 284, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.L.; Mendes, P.M.; Diegoli, S.; Chen, Y.; Preece, J.A.; Palmer, R.E. Electrostatically stabilised nanoparticles: Self-organization and electron-beam patterning. J. Nanosci. Nanotechnol. 2005, 5, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Roller, E.M.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Song, C.; Wang, J.; Shi, D.; Wang, Z.; Liu, N.; Ding, B. Rolling up gold nanoparticle-dressed DNA origami into three-dimensional plasmonic chiral nanostructures. J. Am. Chem. Soc. 2012, 134, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Asenjo-Garcia, A.; Liu, Q.; Jiang, Q.; García de Abajo, F.J.; Liu, N.; Ding, B. Three-dimensional plasmonic chiral tetramers assembled by DNA origami. Nano Lett. 2013, 13, 2128–2133. [Google Scholar] [CrossRef]
- Ou, J.; Tan, H.; Chen, X.; Chen, Z. DNA-Assisted Assembly of Gold Nanostructures and Their Induced Optical Properties. Nanomaterials 2018, 8, 994. [Google Scholar] [CrossRef]
- De Oliveira, F.M.; de Araújo Nascimento, R.L.B.; Calado, C.M.S.; Meneghetti, M.R.; da Silva, M.G.A. Aqueous-Phase Catalytic Chemical Reduction of p-Nitrophenol Employing Soluble Gold Nanoparticles with different shapes. Catalysts 2016, 6, 215. [Google Scholar] [CrossRef]
- Wen, Y.; McLaughlin, C.K.; Lo, P.K.; Yang, H.; Sleiman, H.F. Stable gold nanoparticle conjugation to internal DNA positions: Facile generation of discrete gold nanoparticle–DNA assemblies. Bioconjug. Chem. 2010, 21, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.Y.; Tian, Q.W.; Yang, S.P. Small Gold Nanorods: Recent Advances in Synthesis, Biological Imaging, and Cancer Therapy. Materials 2017, 10, 1372. [Google Scholar] [CrossRef]
- Pal, S.; Deng, Z.; Wang, H.; Zou, S.; Liu, Y.; Yan, H. DNA Directed Self-Assembly of Anisotropic Plasmonic Nanostructures. J. Am. Chem. Soc. 2011, 133, 17606–17609. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Chua, Y.A.; Zhang, W.; Huang, H.; Lu, X. Highly Symmetric Gold Nanostars: Crystallographic Control and Surface-Enhanced Raman Scattering Property. J. Am. Chem. Soc. 2015, 137, 10460–10463. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Donà, A.; Taglietti, A.; Minzioni, P.; Patrini, M.; Dacarro, G.; Chirico, G.; Sironi, L.; Bloise, N.; Visai, L.; et al. Self-assembled monolayers of gold nanostars: A convenient tool for near-IR photothermal biofilm eradication. Chem. Commun. 2014, 50, 1969–1971. [Google Scholar] [CrossRef] [PubMed]
- Dacarro, G.; Pallavicini, P.; Bertani, S.M.; Chirico, G.; D’Alfonso, L.; Falqui, A.; Marchesi, N.; Pascale, A.; Sironi, L.; Taglietti, A.; et al. Synthesis of reduced-size gold nanostars and internalization in SH-SY5Y cells. J. Colloid Interface Sci. 2017, 1, 1055–1064. [Google Scholar] [CrossRef]
- Ma, T.; Yang, W.; Liu, S.; Zhang, H.; Liang, F. A Comparison Reduction of 4-Nitrophenol by Gold Nanospheres and Gold Nanostars. Catalysts 2017, 7, 38. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ricciardi, L.; Deitz, J.I.; Williams, R.E.A.; Mc Comb, D.W.; Strangi, G. Heterodimeric Plasmonic Nanogaps for Biosensing. Micromachines 2018, 9, 664. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, L.; Xu, G. Seed-mediated growth of noble metal nanocrystals: Crystal growth and shape control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Large, N.; Wang, H. Gold nanoparticles with tipped surface structures as substrates for single-particle surface-enhanced Raman spectroscopy: Concave nanocubes, nanotrisoctahedra, and nanostars. ACS Appl. Mater Inter. 2014, 6, 17255–17267. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Kuo, C.H.; Huang, M.H. Seed-Mediated Synthesis of Gold Nanocrystals with Systematic Shape Evolution from Cubic to Trisoctahedral and Rhombic Dodecahedral Structures. Langmuir 2010, 26, 12307–12313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, R.; Niu, R.; Sun, W.; Shao, T.; Chen, X. Gold Nanocage-Based Electrochemical Sensing Platform for Sensitive Detection of Luteolin. Sensors 2018, 18, 2309. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, X.; Wang, Z.; Jin, A.; Sun, X.; Zhu, L.; Wang, F.; Ma, Y.; Niu, G.; Walker, A.R.H.; et al. Gold Nanoparticle-Based Activatable Probe for Sensing Ultralow Levels of Prostate-Specific Antigen. ACS Nano 2013, 7, 5568–5576. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Liu, G.; Lee, S.; Chen, X. High-sensitivity nanosensors for biomarker detection. Chem. Soc. Rev. 2012, 41, 2641–2655. [Google Scholar] [CrossRef]
- Catalán, J.; Norppa, H. Safety Aspects of Bio-Based Nanomaterials. Bioengineering 2017, 4, 94. [Google Scholar] [CrossRef]








| Morphology | Size (nm) | Functionality | Biomedical Investigations | Ref. |
|---|---|---|---|---|
| Nanospheres | ||||
| 20 | acid | cytotoxicity test | [48] | |
| 90 | acid & amine | optical sensors | [4,70] | |
| 10 | sulfonate | drug delivery | [49,59] | |
| 10 | sulfonate | fluorescence resonance energy transfer (FRET) | [62] | |
| 100 | glycol | photothermal therapy (PTT) | [32] | |
| 5 | acid & amine | computed tomography | [35] | |
| 20 | ester | sensor | [86] | |
| 10 | sulfonate | sensor | [36] | |
| 10 | alginate | sensor | [23] | |
| Nanorods | ||||
| 16–50 | acid | cytotoxicity test | [48] | |
| 16–50 | ether | cytotoxicity test | [48] | |
| 100 | acid & amine | optical sensor | [8] | |
| 16–50 | acid & amine | optical sensor | [2] | |
| 38–118 | glycol | dye fluorescence enhancement | [10] | |
| 15–60 | amine | conjugation with Cytochrome C | [28] | |
| 25–35 | acid & amine | dye absorption enhancement | [18] | |
| Nanostars | ||||
| 18–70 | acid & ester | cell transfection | [81] | |
| 50 | amine | singlet oxygen production | [82] | |
| 40 | HEPES-aptamer | anticancer effects | [87] | |
| peptides-PEG | photothermal properties | [88] | ||
| 30 | lauryl sulfobetaine | photothermal properties | [89] | |
| Nanocubes | ||||
| 10–300 | gold/prussian blue analogue | biosensor | [4] | |
| 80 | acid | biosensor | [90] | |
| 5–10 | acid | catalyst | [24] | |
| 100 | amine | imaging | [91] | |
| 50–80 | amine | biosensor | [92] | |
| Hollow nanoparticles | ||||
| 80–100 | pyridinium | drug delivery | [77] | |
| 200–1000 | acid & ester | drug delivery | [79] | |
| 50 | acid & ester | photothermal properties | [84] | |
| Nanocapsules | ||||
| 40 | acid & ester | optical sensitivity | [75] | |
| 50 | acid | photothermal properties | [76] | |
| 30 | dye | imaging | [78] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venditti, I. Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioengineering 2019, 6, 53. https://doi.org/10.3390/bioengineering6020053
Venditti I. Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioengineering. 2019; 6(2):53. https://doi.org/10.3390/bioengineering6020053
Chicago/Turabian StyleVenditti, Iole. 2019. "Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review" Bioengineering 6, no. 2: 53. https://doi.org/10.3390/bioengineering6020053
APA StyleVenditti, I. (2019). Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioengineering, 6(2), 53. https://doi.org/10.3390/bioengineering6020053