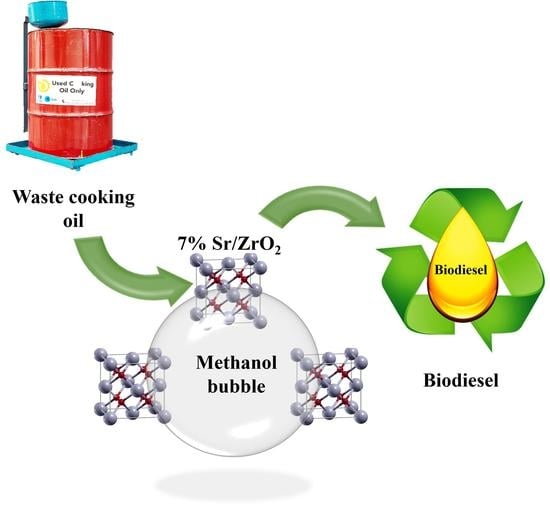
Intensification of Biodiesel Processing from Waste Cooking Oil, Exploiting Cooperative Microbubble and Bifunctional Metallic Heterogeneous Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Purification
2.3. Catalyst Preparation
2.4. Characterization of Catalyst
2.5. Experimental Procedure
2.6. Biodiesel Analysis
3. Results and Discussion
3.1. Catalyst Analysis
3.2. Parameter Optimization for Biodiesel Production
3.2.1. Effect of Molar Ratio on Biodiesel Production
3.2.2. Influence of Catalyst on the Conversion of WCO
3.2.3. Effect of Temperature on Biodiesel Production
3.2.4. Effect of Reaction Time on Biodiesel Production
| Catalyst | Temperature (°C) | Time (min) | Catalyst Loading (wt.%) | Conversion (%) | Reference |
|---|---|---|---|---|---|
| Li-Al HTA | 65 | 60 | 3 | 83 | [35] |
| KI/SiO | 70 | 480 | 5 | 91 | [36] |
| KF/Al2O3 | 60 | 480 | 3 | 90 | [37] |
| 3% La2O3–ZrO2 | 65 | 300 | 6 | 56 | [38] |
| ZrO2/SiO2 | 120 | 120 | 10 | 48.6 | [6] |
| Li/ZrO2 | 65 | 180 | 3 | 98.2 | [39] |
| 21% La2O3/ZrO2 | 200 | 480 | 5 | 84.9 | [40] |
| 7% Sr/ZrO2 | 70 | 20 | 1 | 85 | This study |
3.3. Reaction Kinetics and Mechanism of WCO-Based Biodiesel
3.3.1. Proposal of a Reaction Mechanism for Biodiesel Production Using 7% SR/ZRO2
3.3.2. Kinetics Analysis and Activation Energy of WCO-Based Biodiesel
| Feedstock | Type of Transesterification | Catalyst | Activation Energy (kJ.mol−1) | Reference |
|---|---|---|---|---|
| Waste cooking oil | Ultra-Sonication | Calcium diglyceroxide | 119.23 | [34] |
| Waste cooking oil | Supercritical method | No catalyst | 50.5 | [47] |
| Waste cooking oil | Microwave technology | Calcium diglyceroxide | 26.56 | [48] |
| Waste cooking oil | Conventional method | CaO/SiO2 | 66.27 | [49] |
| Waste cooking oil | Conventional method | Cs2.5H0.5PW12O40 | 36 | [50] |
| Stearic acid | Conventional method | ZrO2/SiO2 | 47 | [51] |
| Rapeseed oil | Solvent-free method | Sulfated zirconia | 22.5 | [52] |
| Levulinic acid | Conventional method | SO42−/ZrO2 | 14.61 | [53] |
| Oleic acid | Microbubble process | H2SO4 | 26.37 | [27] |
| Chicken fat oil | Microbubble process | PTSA | 24.9 | [28] |
| Waste cooking oil | Microbubble process | 7% Sr/ZrO2 | 7.4 | This study |
3.4. Reusability and Reactivation of the Sr/ZrO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, R.; Romero-Ibarra, I.; Vazquez-Arenas, J. Synthesis of Sodium Zincsilicate (Na2ZnSiO4) and heterogeneous catalysis towards biodiesel production via Box-Behnken Design. Fuel 2020, 280, 118668. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mahmoud, H.R.; El-Molla, S.A. Influence of support on physicochemical properties of ZrO2 based solid acid heterogeneous catalysts for biodiesel production. Catal. Commun. 2019, 122, 10–15. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.; Madzimbamuto, T.; Ikhu-Omoregbe, D. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Al-Sakkari, E.; El-Sheltawy, S.; Attia, N.; Mostafa, S. Kinetic study of soybean oil methanolysis using cement kiln dust as a heterogeneous catalyst for biodiesel production. Appl. Catal. B Environ. 2017, 206, 146–157. [Google Scholar] [CrossRef]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- de Lima, A.L.; Ronconi, C.M.; Mota, C.J. Heterogeneous basic catalysts for biodiesel production. Catal. Sci. Technol. 2016, 6, 2877–2891. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Chai, M.; Tu, Q.; Lu, M.; Yang, Y.J. Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel Process. Technol. 2014, 125, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Rashid, U.; Taufiq-Yap, Y. Synthesis of waste cooking oil-based biodiesel via effectual recyclable bi-functional Fe2O3MnOSO42−/ZrO2 nanoparticle solid catalyst. Fuel 2015, 142, 38–45. [Google Scholar] [CrossRef]
- Ranjbar, M.; Yousefi, M.; Lahooti, M.; Malekzadeh, A. Preparation and characterization of tetragonal zirconium oxide nanocrystals from isophthalic acid-zirconium (IV) nanocomposite as a new precursor. Int. J. Nanosci. Nanotechnol. 2012, 8, 191–196. [Google Scholar]
- Li, H.; Fang, Z.; Smith, R.L., Jr.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Raia, R.Z.; da Silva, L.S.; Marcucci, S.M.P.; Arroyo, P.A. Biodiesel production from Jatropha curcas L. oil by simultaneous esterification and transesterification using sulphated zirconia. Catal. Today 2017, 289, 105–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, L.; Esmaeili, H. A review on biodiesel production using various heterogeneous nanocatalysts: Operation mechanisms and performances. Biomass Bioenergy 2022, 158, 106356. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Jamil, F.; Myint, M.T.Z.; Al-Hinai, M.; Al-Haj, L.; Baawain, M.; Al-Abri, M.; Kumar, G.; Atabani, A. Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@ MgO-ZrO2). Energy Convers. Manag. 2018, 155, 128–137. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Tesar, V.; Butler, S.; Bandulasena, H.C. Microbubble generation. Recent Pat. Eng. 2008, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, W.B.; Al-Mashhadani, M.K.; Bandulasena, H.H. Evaporation dynamics of microbubbles. Chem. Eng. Sci. 2013, 101, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, W.B.J.; Tesar, V. Bubble generation for aeration and other purposes. U.S. Patent No EP2081666B1, 19 October 2011. [Google Scholar]
- Ahmad, N.; Javed, F.; Awan, J.A.; Ali, S.; Fazal, T.; Hafeez, A.; Aslam, R.; Rashid, N.; Rehman, M.S.U.; Zimmerman, W.B. Biodiesel production intensification through microbubble mediated esterification. Fuel 2019, 253, 25–31. [Google Scholar] [CrossRef]
- Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, R.; Akram, S.; Rashid, N.; Zimmerman, W.B.; Rehman, F. Conversion of poultry-fat waste to a sustainable feedstock for biodiesel production via microbubble injection of reagent vapor. J. Clean. Prod. 2021, 311, 127525. [Google Scholar] [CrossRef]
- Hanotu, J.O.; Bandulasena, H.; Zimmerman, W.B. Aerator design for microbubble generation. Chem. Eng. Res. Des. 2017, 123, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Hanotu, J.; Bandulasena, H.H.; Chiu, T.Y.; Zimmerman, W.B. Oil emulsion separation with fluidic oscillator generated microbubbles. Int. J. Multiph. Flow 2013, 56, 119–125. [Google Scholar] [CrossRef]
- Jothinathan, L.; Cai, Q.; Ong, S.; Hu, J. Fe-Mn doped powdered activated carbon pellet as ozone catalyst for cost-effective phenolic wastewater treatment: Mechanism studies and phenol by-products elimination. J. Hazard. Mater. 2022, 424, 127483. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Catalytic ozonation of dye in a microbubble system: Hydroxyl radical contribution and effect of salt. J. Environ. Chem. Eng. 2016, 4, 2250–2258. [Google Scholar] [CrossRef]
- Li, X.-F.; Zuo, Y.; Zhang, Y.; Fu, Y.; Guo, Q.-X. In situ preparation of K2CO3 supported Kraft lignin activated carbon as solid base catalyst for biodiesel production. Fuel 2013, 113, 435–442. [Google Scholar] [CrossRef]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Shumaker, J.L.; Crofcheck, C.; Tackett, S.A.; Santillan-Jimenez, E.; Crocker, M. Biodiesel production from soybean oil using calcined Li–Al layered double hydroxide catalysts. Catal. Lett. 2007, 115, 56–61. [Google Scholar] [CrossRef]
- Samart, C.; Sreetongkittikul, P.; Sookman, C. Heterogeneous catalysis of transesterification of soybean oil using KI/mesoporous silica. Fuel Process. Technol. 2009, 90, 922–925. [Google Scholar] [CrossRef]
- Boz, N.; Kara, M.; Sunal, O.; Alptekin, E.; Değirmenbaşi, N. Investigation of the fuel properties of biodiesel produced over an alumina-based solid catalyst. Turk. J. Chem. 2009, 33, 433–442. [Google Scholar] [CrossRef]
- Salinas, D.; Sepúlveda, C.; Escalona, N.; Gfierro, J.; Pecchi, G. Sol–gel La2O3–ZrO2 mixed oxide catalysts for biodiesel production. J. Energy Chem. 2018, 27, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Sun, H.; Duan, J.; Chen, P.; Lou, H.; Zheng, X. Mesoporous Li/ZrO2 as a solid base catalyst for biodiesel production from transesterification of soybean oil with methanol. Catal. Commun. 2011, 12, 606–610. [Google Scholar] [CrossRef]
- Georgogianni, K.; Katsoulidis, A.; Pomonis, P.; Manos, G.; Kontominas, M. Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process. Technol. 2009, 90, 1016–1022. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Kierzkowska-Pawlak, H. Determination of kinetics in gas-liquid reaction systems. An overview. Ecol. Chem. Eng. S 2012, 19, 175–196. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.; Vega, A.; Coca, J. Correlation for the estimation of gas-liquid diffusivity. Chem. Eng. Commun. 1987, 52, 271–281. [Google Scholar] [CrossRef]
- Kimweri, H.T.H. Enhancement of Gas-Liquid Mass Transfer in Hydrometallurgical Leaching Systems; University of British Columbia: Vancouver, BC, Canada, 2001. [Google Scholar]
- Neumann, K.; Werth, K.; Martín, A.; Górak, A. Biodiesel production from waste cooking oils through esterification: Catalyst screening, chemical equilibrium and reaction kinetics. Chem. Eng. Res. Des. 2016, 107, 52–62. [Google Scholar] [CrossRef]
- Roman, F.F.; Ribeiro, A.E.; Queiroz, A.; Lenzi, G.G.; Chaves, E.S.; Brito, P. Optimization and kinetic study of biodiesel production through esterification of oleic acid applying ionic liquids as catalysts. Fuel 2019, 239, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Biodiesel production from waste cooking oil via supercritical methanol: Optimisation and reactor simulation. Renew. Energy 2018, 124, 144–154. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew. Energy 2018, 121, 757–767. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Li, L.; Zou, C.; Zhou, L.; Lin, L. Cucurbituril-protected Cs2.5H0.5PW12O40 for optimized biodiesel production from waste cooking oil. Renew. Energy 2017, 107, 14–22. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; El-Molla, S.A.; Ibrahim, M.M. Biodiesel production via stearic acid esterification over mesoporous ZrO2/SiO2 catalysts synthesized by surfactant-assisted sol-gel auto-combustion route. Renew. Energy 2020, 160, 42–51. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Harvey, A.P.; Thanapimmetha, A.; Srinophakun, P. Kinetic of myristic acid esterification with methanol in the presence of triglycerides over sulfated zirconia. Renew. Energy 2011, 36, 2679–2686. [Google Scholar] [CrossRef]
- Unlu, D.; Ilgen, O.; Hilmioglu, N.D. Biodiesel additive ethyl levulinate synthesis by catalytic membrane: SO4−2/ZrO2 loaded hydroxyethyl cellulose. Chem. Eng. J. 2016, 302, 260–268. [Google Scholar] [CrossRef]
- Jabeen, M.; Munir, M.; Abbas, M.M.; Ahmad, M.; Waseem, A.; Saeed, M.; Kalam, M.A.; Zafar, M.; Sultana, S.; Mohamed, A. Sustainable production of biodiesel from novel and non-edible ailanthus altissima (Mill.) seed oil from green and recyclable potassium hydroxide activated ailanthus cake and cadmium sulfide catalyst. Sustainability 2022, 14, 10962. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Mubashir, M.; Asif, S.; Waseem, A.; Mukhtar, A.; Saqib, S.; Munawaroh, H.S.H.; Lam, M.K.; Khoo, K.S. A practical approach for synthesis of biodiesel via non-edible seeds oils using trimetallic based montmorillonite nano-catalyst. Bioresour. Technol. 2021, 328, 124859. [Google Scholar] [CrossRef] [PubMed]









| Parameters | Units | Values |
|---|---|---|
| Viscosity | mPa·s | 31.16 |
| Density | kg·m−3 | 919 |
| FFA | % | 9 |
| Oil composition | ||
| Linoleic acid | wt.% | 9 |
| Linolenic acid | wt.% | 62 |
| Palmitic acid | wt.% | 12 |
| Lignoceric acid | wt.% | 17 |
| Catalyst | Surface Area (m2/g) | BJH Area (m2/g) | BJH Volume (cm3/g) | BET Pore Diameter (nm) | BJH Pore Diameter (nm) |
|---|---|---|---|---|---|
| 7% Sr/ZrO2 | 119.80 | 117.34 | 0.2154 | 8.97 | 8.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, F.; Rizwan, M.; Asif, M.; Ali, S.; Aslam, R.; Akram, M.S.; Zimmerman, W.B.; Rehman, F. Intensification of Biodiesel Processing from Waste Cooking Oil, Exploiting Cooperative Microbubble and Bifunctional Metallic Heterogeneous Catalysis. Bioengineering 2022, 9, 533. https://doi.org/10.3390/bioengineering9100533
Javed F, Rizwan M, Asif M, Ali S, Aslam R, Akram MS, Zimmerman WB, Rehman F. Intensification of Biodiesel Processing from Waste Cooking Oil, Exploiting Cooperative Microbubble and Bifunctional Metallic Heterogeneous Catalysis. Bioengineering. 2022; 9(10):533. https://doi.org/10.3390/bioengineering9100533
Chicago/Turabian StyleJaved, Fahed, Muhammad Rizwan, Maryam Asif, Shahzad Ali, Rabya Aslam, Muhammad Sarfraz Akram, William B Zimmerman, and Fahad Rehman. 2022. "Intensification of Biodiesel Processing from Waste Cooking Oil, Exploiting Cooperative Microbubble and Bifunctional Metallic Heterogeneous Catalysis" Bioengineering 9, no. 10: 533. https://doi.org/10.3390/bioengineering9100533
APA StyleJaved, F., Rizwan, M., Asif, M., Ali, S., Aslam, R., Akram, M. S., Zimmerman, W. B., & Rehman, F. (2022). Intensification of Biodiesel Processing from Waste Cooking Oil, Exploiting Cooperative Microbubble and Bifunctional Metallic Heterogeneous Catalysis. Bioengineering, 9(10), 533. https://doi.org/10.3390/bioengineering9100533