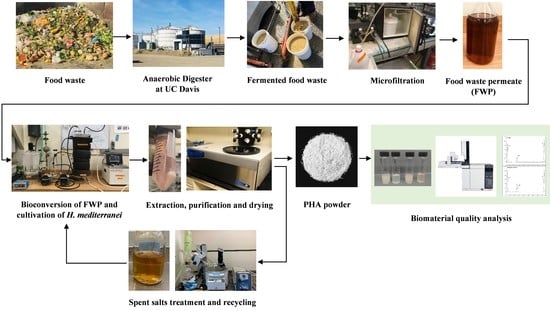
Process Development of Polyhydroxyalkanoates Production by Halophiles Valorising Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Collection and Pretreatment
2.2. Microorganism and Culture Conditions
2.3. Batch Production System with Two Aeration Strategies
2.4. Spent Saline Medium Treatment and Recycling
2.5. Analytical Methods
2.5.1. Feedstock Characterization
2.5.2. Cell Biomass Measurement
2.5.3. Product Extraction and Quantification
2.6. Statistical Analysis
3. Results
3.1. Characterization of Microfiltration Streams
3.2. Effect of Aeration Rate
3.3. Effects of Substrate Loading and Type
3.4. Batch Production in Integrated Bioreactor System
3.5. Effect of Spent Saline Medium Recycling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, C.S.K.; Ghai, R.; Rashmi; Kalia, V.C. Polyhydroxyalkanoates: An Overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Chen, G.Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Rahman, A.; Rehman, A.; Walsh, M.; Miller, C. Food Waste Conversion to Microbial Polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hobby, A.M.; Chen, Y.; Chio, A.; Jenkins, B.M.; Zhang, R. Techno-Economic Analysis on an Industrial-Scale Production System of Polyhydroxyalkanoates (PHA) from Cheese By-Products by Halophiles. Processes 2022, 10, 17. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R. Production of Polyhydroxyalkanoates (PHA) by Haloferax mediterranei from Food Waste Derived Nutrients for Biodegradable Plastic Applications. J. Microbiol. Biotechnol. 2021, 31, 338–347. [Google Scholar] [CrossRef]
- Campanari, S.; Augelletti, F.; Rossetti, S.; Sciubba, F.; Villano, M.; Majone, M. Enhancing a Multi-Stage Process for Olive Oil Mill Wastewater Valorization towards Polyhydroxyalkanoates and Biogas Production. Chem. Eng. J. 2017, 317, 280–289. [Google Scholar] [CrossRef]
- Colombo, B.; Pepè Sciarria, T.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) Production from Fermented Cheese Whey by Using a Mixed Microbial Culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Hall, P.V.; Darby, J.L.; Coats, E.R.; Green, P.G.; Thompson, D.E.; Loge, F.J. Production of Polyhydroxyalkanoate During Treatment of Tomato Cannery Wastewater. Water Environ. Res. 2008, 80, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Hu, H.; Ji, H.; Cai, J.; He, N.; Li, Q.; Wang, Y. Production of Poly(Hydroxybutyrate–Hydroxyvalerate) from Waste Organics by the Two-Stage Process: Focus on the Intermediate Volatile Fatty Acids. Bioresour. Technol. 2014, 166, 194–200. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Jana, K.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; De, S.; Mukherjee, J. Integration of Poly-3-(Hydroxybutyrate-Co-Hydroxyvalerate) Production by Haloferax mediterranei through Utilization of Stillage from Rice-Based Ethanol Manufacture in India and Its Techno-Economic Analysis. World J. Microbiol. Biotechnol. 2015, 31, 717–727. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Biosynthesis and Characterization of Polyhydroxyalkanoates with Controlled Composition and Microstructure. Biomacromolecules 2018, 19, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Quillaguamán, J.; Guzmán, H.; Van-Thuoc, D.; Hatti-Kaul, R. Synthesis and Production of Polyhydroxyalkanoates by Halophiles: Current Potential and Future Prospects. Appl. Microbiol. Biotechnol. 2010, 85, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, Z.-B.; Sheng, G.-P.; Yu, H.-Q. Kinetic Analysis on the Production of Polyhydroxyalkanoates from Volatile Fatty Acids by Cupriavidus Necator with a Consideration of Substrate Inhibition, Cell Growth, Maintenance, and Product Formation. Biochem. Eng. J. 2010, 49, 422–428. [Google Scholar] [CrossRef]
- Ghosh, S.; Gnaim, R.; Greiserman, S.; Fadeev, L.; Gozin, M.; Golberg, A. Macroalgal Biomass Subcritical Hydrolysates for the Production of Polyhydroxyalkanoate (PHA) by Haloferax mediterranei. Bioresour. Technol. 2019, 271, 166–173. [Google Scholar] [CrossRef] [PubMed]
- DEP DO Saturation Calculator|Florida Department of Environmental Protection. Available online: https://floridadep.gov/dear/water-quality-standards-program/documents/do-saturation-calculator%C2%A0 (accessed on 24 September 2021).
- Cui, Y.-W.; Shi, Y.-P.; Gong, X.-Y. Effects of C/N in the Substrate on the Simultaneous Production of Polyhydroxyalkanoates and Extracellular Polymeric Substances by Haloferax mediterranei via Kinetic Model Analysis. RSC Adv. 2017, 7, 18953–18961. [Google Scholar] [CrossRef] [Green Version]
- Pais, J.; Serafim, L.S.; Freitas, F.; Reis, M.A.M. Conversion of Cheese Whey into Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Haloferax mediterranei. New Biotechnol. 2016, 33, 224–230. [Google Scholar] [CrossRef]
- Lillo, J.G.; Rodriguez-Valera, F. Effects of Culture Conditions on Poly(3-Hydroxybutyric Acid) Production by Haloferax mediterranei. Appl. Environ. Microbiol. 1990, 56, 2517–2521. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of High Quality Polyhydroxyalkanoate Co- and Terpolyesters for Potential Medical Application by the Archaeon Haloferax mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Koller, M. Recycling of Waste Streams of the Biotechnological Poly(Hydroxyalkanoate) Production by Haloferax mediterranei on Whey. Int. J. Polym. Sci. 2015, 2015, e370164. [Google Scholar] [CrossRef] [Green Version]
- Lorantfy, B.; Seyer, B.; Herwig, C. Stoichiometric and Kinetic Analysis of Extreme Halophilic Archaea on Various Substrates in a Corrosion Resistant Bioreactor. New Biotechnol. 2014, 31, 80–89. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Indicators: Salinity. Available online: https://www.epa.gov/national-aquatic-resource-surveys/indicators-salinity (accessed on 15 April 2019).
- U.S. Environmental Protection Agency. Water Quality Standards: Regulations and Resources. Available online: https://www.epa.gov/wqs-tech (accessed on 15 April 2019).
- Bhattacharyya, A.; Saha, J.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; Mukherjee, J. Production of Poly-3-(Hydroxybutyrate-Co-Hydroxyvalerate) by Haloferax mediterranei Using Rice-Based Ethanol Stillage with Simultaneous Recovery and Re-Use of Medium Salts. Extremophiles 2014, 18, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Nie, J.; Wu, S.; Wang, D. Removal of COD and Decolorizing from Landfill Leachate by Fenton’s Reagent Advanced Oxidation. Clean Technol. Environ. Policy 2014, 16, 189–193. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D.; Muresan, A.; Muresan, R.; Popescu, A. Textile Wastewater Treatment by Homogenous Oxidation with Hydrogen Peroxide. Environ. Eng. Manag. J. 2009, 8, 1359–1369. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of Food Waste as Feedstock for Anaerobic Digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef]
- Dyall-Smith, M. The Halohandbook Protocols for Halobacterial Genetics, Version 7.2; Martinsried, Germany, 2009; p. 144. Available online: https://haloarchaea.com/halohandbook/ (accessed on 17 June 2018).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Escalona, A.M.; Varela, F.R.; Gomis, A.M. Procedure for Extraction of Polyhydroxyalkanoates from Halophilic Bacteria Which Contain Them. U.S. Patent 5,536,419, 16 July 1996. [Google Scholar]
- Yu, J.; Si, Y.; Keung, W.; Wong, R. Kinetics Modeling of Inhibition and Utilization of Mixed Volatile Fatty Acids in the Formation of Polyhydroxyalkanoates by Ralstonia Eutropha. Process Biochem. 2002, 37, 731–738. [Google Scholar] [CrossRef]
- Doran, P.M. Bioprocess Development. In Bioprocess Engineering Principles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–11. ISBN 978-0-12-220851-5. [Google Scholar]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor Scale-up and Oxygen Transfer Rate in Microbial Processes: An Overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Process Analysis and Economic Evaluation for Poly(3-Hydroxybutyrate) Production by Fermentation. Bioprocess Eng. 1997, 17, 335–342. [Google Scholar] [CrossRef]
- Leong, Y.K.; Show, P.L.; Lan, J.C.-W.; Loh, H.-S.; Lam, H.L.; Ling, T.C. Economic and Environmental Analysis of PHAs Production Process. Clean Technol. Environ. Policy 2017, 19, 1941–1953. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A One-Stage Cultivation Process for the Production of Poly-3-(Hydroxybutyrate-Co-Hydroxyvalerate) from Olive Mill Wastewater by Haloferax mediterranei. New Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Production of the Copolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Varied Composition Using Different Nitrogen Sources with Haloferax mediterranei. Extremophiles 2017, 21, 1037–1047. [Google Scholar] [CrossRef]
- Wang, K. Process Development of Polyhydroxyalkanoates Production by Halophiles Valorizing Municipal Food Waste with Spent Salts Recycling: Datasets and Supplemental Materials. 2022. Available online: https://data.mendeley.com/datasets/r38mjyvfgr (accessed on 9 August 2022).






| Food Waste 1 | Food Waste 2 | ||||||
|---|---|---|---|---|---|---|---|
| Compound | Unit | Influent 1 | Permeate 1 | Retentate 1 | Influent 2 | Permeate 2 | Retentate 2 |
| Lactic acid | g/L | 24.7 ± 0.9 | 21.5 ± 1.3 | 20.4 ± 0.6 | 37.8 ± 0.4 | 36.6 ± 0.5 | 36.0 ± 0.4 |
| Acetic acid | g/L | 10.1 ± 0.8 | 8.7 ± 0.6 | 8.3 ± 0.4 | 17.7 ± 0.2 | 16.8 ± 0.1 | 15.9 ± 0.1 |
| Propionic acid | g/L | 20.0 ± 1.2 | 17.2 ± 1.1 | 16.7 ± 0.6 | 14.1 ± 0.2 | 12.9 ± 0.2 | 12.2 ± 0.1 |
| Butyric acid | g/L | 4.4 ± 0.3 | 3.8 ± 0.3 | 2.8 ± 0.1 | 4.4 ± 0.1 | 4.1 ± 0.1 | 3.6 ± 0.1 |
| Iso-butyric acid | g/L | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Iso-valeric acid | g/L | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.0 |
| Valeric acid | g/L | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Total carboxylic acids | g/L | 60.2 ± 3.5 | 52.4 ± 3.4 | 49.7 ± 2.8 | 76.2 ± 0.5 | 71.6 ± 0.7 | 68.8 ± 0.4 |
| COD from acids | g/L | 82.5 ± 1.5 | 71.5 ± 1.7 | 71.1 ± 2.0 | 96.5 ± 1.0 | 84.8 ± 0.5 | 83.9 ± 0.5 |
| Total COD | g/L | 232.6 ± 1.5 | 108.5 ± 2.0 | 560.3 ± 7.3 | 108.5 ± 0.8 | 92.5 ± 1.0 | 149.8 ± 1.2 |
| Total N | g/L N | 4.6 ± 0.3 | 3.8 ± 0.1 | 6.7 ± 0.4 | 3.0 ± 0.2 | 2.5 ± 0.1 | 4.3 ± 0.3 |
| Total P | g/L P | 3.7 ± 0.1 | 0.8 ± 0.0 | 6.5 ± 0.1 | 2.8 ± 0.1 | 0.6 ± 0.0 | 4.8 ± 0.2 |
| Compound | Unit | Permeate 1 | Permeate 2 | MSM and SL-6 |
|---|---|---|---|---|
| N | g/L | 3.8 ± 0.1 | 2.5 ± 0.1 | 0 |
| P | g/L | 0.8 ± 0.0 | 0.6 ± 0.0 | 0 |
| K | mg/L | 2055 ± 7 | 2072 ± 5 | 1950 |
| Ca | mg/L | 1588 ± 3 | 1629 ± 6 | 183 |
| Mg | mg/L | 202 ± 0 | 198 ± 2 | 5346 |
| Na | mg/L | 1579 ± 2 | 1485 ± 1 | 61,410 |
| B | mg/L | 1.69 ± 0.00 | 1.62 ± 0.01 | 0.5 |
| Zn | mg/L | 3.79 ± 0.01 | 3.83 ± 0.01 | 0.25 |
| Cu | mg/L | 0.50 ± 0.01 | 0.38 ± 0.00 | 0.04 |
| Mn | mg/L | 2.81 ± 0.00 | 2.92 ± 0.01 | 0.13 |
| Fe | mg/L | 30.80 ± 0.71 | 30.52 ± 0.65 | 17.3 |
| Ni | mg/L | 0.20 ± 0.00 | 0.18 ± 0.00 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Chen, C.; Zhang, R. Process Development of Polyhydroxyalkanoates Production by Halophiles Valorising Food Waste. Bioengineering 2022, 9, 630. https://doi.org/10.3390/bioengineering9110630
Wang K, Chen C, Zhang R. Process Development of Polyhydroxyalkanoates Production by Halophiles Valorising Food Waste. Bioengineering. 2022; 9(11):630. https://doi.org/10.3390/bioengineering9110630
Chicago/Turabian StyleWang, Ke, Chang Chen, and Ruihong Zhang. 2022. "Process Development of Polyhydroxyalkanoates Production by Halophiles Valorising Food Waste" Bioengineering 9, no. 11: 630. https://doi.org/10.3390/bioengineering9110630
APA StyleWang, K., Chen, C., & Zhang, R. (2022). Process Development of Polyhydroxyalkanoates Production by Halophiles Valorising Food Waste. Bioengineering, 9(11), 630. https://doi.org/10.3390/bioengineering9110630