Recombinant Expression of Human IL-33 Protein and Its Effect on Skin Wound Healing in Diabetic Mice
Abstract
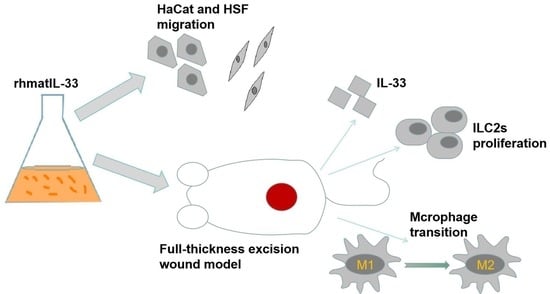
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of rhmatIL-33 Protein in Escherichia coli
2.2. Cell Migration Assay
2.3. Full-Thickness Excision Wound Model in Mice
2.4. Wound Assessment
2.5. Real-Time PCR
2.6. Flow Cytometry Assay
2.7. Statistical Analysis
3. Results
3.1. Expression and Purification of rhmatIL-33 Protein
3.2. Effects of rhmatIL-33 on the Migration of HSF and HaCaT Cells
3.3. Effect of rhmatIL-33 on Skin Wound Healing in Mice
3.4. Expression of Endogenous IL-33 in Skin Wounds
3.5. Effects of rhmatIL-33 Administration on ILC2 Cells in Wound Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.-Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Short, W.D.; Wang, X.; Li, H.; Yu, L.; Kaul, A.; Calderon, G.A.; Gilley, J.; Bollyky, P.L.; Balaji, S.; Keswani, S.G. Interleukin-10 Producing T Lymphocytes Attenuate Dermal Scarring. Ann. Surg. 2021, 274, 627. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 02148. [Google Scholar] [CrossRef] [PubMed]
- Doersch, K.M.; DelloStritto, D.J.; Newell-Rogers, M.K. The contribution of interleukin-2 to effective wound healing. Exp. Biol. Med. 2017, 242, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Loots, M.A.; Kenter, S.B.; Au, F.L.; Van Galen, W.; Middelkoop, E.; Bos, J.D.; Mekkes, J.R. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur. J. Cell Biol. 2002, 81, 153–160. [Google Scholar] [CrossRef]
- Öhnstedt, E.; Tomenius, H.L.; Vågesjö, E.; Phillipson, M. The discovery and development of topical medicines for wound healing. Expert Opin. Drug Discov. 2019, 14, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.J.; Melvin, W.J.; Gallagher, K. Macrophage-mediated inflammation in diabetic wound repair. Semin. Cell Dev. Biol. 2021, 199, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The treatment of impaired wound healing in diabetes: Looking among old drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- De la Fuente, M.; MacDonald, T.T.; Hermoso, M.A. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. 2015, 26, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Vocca, L.; Di Sano, C.; Uasuf, C.G.; Sala, A.; Riccobono, L.; Gangemi, S.; Albano, G.D.; Bonanno, A.; Gagliardo, R.; Profita, M. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology 2015, 220, 954–963. [Google Scholar] [CrossRef] [PubMed]
- de Kleer, I.M.; Kool, M.; de Bruijn, M.J.; Willart, M.; Van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; Afonina, I.S.; Mueller, C.; Beyaert, R. Dichotomous function of IL-33 in health and disease: From biology to clinical implications. Biochem. Pharmacol. 2018, 148, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Sponheim, J.; Pollheimer, J.; Olsen, T.; Balogh, J.; Hammarström, C.; Loos, T.; Kasprzycka, M.; Sørensen, D.R.; Nilsen, H.R.; Küchler, A.M. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am. J. Pathol. 2010, 177, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, X.; Hu, S.; Liu, T.; Yuan, B.; Gu, H.; Ni, Q.; Zhang, X.; Zheng, F. IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol. Immunol. 2013, 56, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Yang, M.; Guo, X.; Zhang, M.; Li, H.; Li, J.; Zhao, J. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget 2016, 7, 33649. [Google Scholar] [CrossRef]
- Nascimento, D.C.; Melo, P.H.; Pineros, A.R.; Ferreira, R.G.; Colón, D.F.; Donate, P.B.; Castanheira, F.V.; Gozzi, A.; Czaikoski, P.G.; Niedbala, W. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, X.; Zhang, D.; Wang, Y.; Hu, X.; Xu, F.; Jin, M.; Cao, F.; Xu, L. Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J. Neuroinflammation 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Hu, M.-H.; Hsiao, Y.-P.; Wang, Y.-C. ST2 signaling in the tumor microenvironment. Tumor Microenviron. 2020, 1240, 83–93. [Google Scholar] [CrossRef]
- He, R.; Yin, H.; Yuan, B.; Liu, T.; Luo, L.; Huang, P.; Dai, L.; Zeng, K. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol. Immunol. 2017, 90, 42–49. [Google Scholar] [CrossRef]
- Michalik, M.; Wójcik-Pszczoła, K.; Paw, M.; Wnuk, D.; Koczurkiewicz, P.; Sanak, M.; Pękala, E.; Madeja, Z. Fibroblast-to-myofibroblast transition in bronchial asthma. Cell. Mol. Life Sci. 2018, 75, 3943–3961. [Google Scholar] [CrossRef] [PubMed]
- Bullard, K.M.; Longaker, M.T.; Lorenz, H.P. Fetal wound healing: Current biology. World J. Surg. 2003, 27, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Tang, J.; Liu, H.; Shen, C.; Rong, M.; Zhang, Z.; Lai, R. A potential wound-healing-promoting peptide from salamander skin. FASEB J. 2014, 28, 3919–3929. [Google Scholar] [CrossRef]
- Liu, H.; Mu, L.; Tang, J.; Shen, C.; Gao, C.; Rong, M.; Zhang, Z.; Liu, J.; Wu, X.; Yu, H. A potential wound healing-promoting peptide from frog skin. Int. J. Biochem. Cell Biol. 2014, 49, 32–41. [Google Scholar] [CrossRef]
- Rak, G.D.; Osborne, L.C.; Siracusa, M.C.; Kim, B.S.; Wang, K.; Bayat, A.; Artis, D.; Volk, S.W. IL-33-dependent group 2 innate lymphoid cells promote cutaneous wound healing. J. Investig. Dermatol. 2016, 136, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Barna, L.; Chenault, V.M.; Waynant, R.W.; Ilev, I.K.; Longo, L.; Miracco, C.; Johnson, B.; Anders, J.J. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed. Laser Ther. 2004, 22, 281–290. [Google Scholar] [CrossRef]
- Lau, T.; Sahota, D.; Lau, C.; Chan, C.; Lam, F.; Ho, Y.; Fung, K.; Lau, C.; Leung, P. An in vivo investigation on the wound-healing effect of two medicinal herbs using an animal model with foot ulcer. Eur. Surg. Res. 2008, 41, 15–23. [Google Scholar] [CrossRef]
- You, Y.; Zhang, X.; Wang, X.; Yue, D.; Meng, F.; Zhu, J.; Wang, Y.; Sun, X. ILC2 proliferated by IL-33 stimulation alleviates acute colitis in Rag1-/-mouse through promoting M2 macrophage polarization. J. Immunol. Res. 2020, 2020, 5018975. [Google Scholar] [CrossRef]
- Avitabile, S.; Odorisio, T.; Madonna, S.; Eyerich, S.; Guerra, L.; Eyerich, K.; Zambruno, G.; Cavani, A.; Cianfarani, F. Interleukin-22 promotes wound repair in diabetes by improving keratinocyte pro-healing functions. J. Investig. Dermatol. 2015, 135, 2862–2870. [Google Scholar] [CrossRef]
- Oshio, T.; Komine, M.; Tsuda, H.; Tominaga, S.-I.; Saito, H.; Nakae, S.; Ohtsuki, M. Nuclear expression of IL-33 in epidermal keratinocytes promotes wound healing in mice. J. Dermatol. Sci. 2017, 85, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, X.; Hu, S.; Liu, T.; Yuan, B.; Ni, Q.; Lan, F.; Luo, X.; Gu, H.; Zheng, F. IL-33 promotes Staphylococcus aureus-infected wound healing in mice. Int. Immunopharmacol. 2013, 17, 432–438. [Google Scholar] [CrossRef]
- Wu, Y.; Quan, Y.; Liu, Y.; Liu, K.; Li, H.; Jiang, Z.; Zhang, T.; Lei, H.; Radek, K.A.; Li, D.; et al. Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat. Commun. 2016, 7, 13393. [Google Scholar] [CrossRef]
- Rui, T.; Zhang, J.; Xu, X.; Yao, Y.; Kao, R.; Martin, C.M. Reduction in IL-33 expression exaggerates ischaemia/reperfusion-induced myocardial injury in mice with diabetes mellitus. Cardiovasc. Res. 2012, 94, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lin, D.; Sheng, J.; Xie, Y. Intrauterine hyperglycemia induces liver inflammation in mouse male offspring. Int. Immunopharmacol. 2021, 99, 107974. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33–independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra116. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Zhu, W.; Lönnblom, E.; Förster, M.; Johannesson, M.; Tao, P.; Meng, L.; Lu, S.; Holmdahl, R. Natural polymorphism of Ym1 regulates pneumonitis through alternative activation of macrophages. Sci. Adv. 2020, 6, eaba9337. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lin, S.; Xiong, S.; Xie, Q. Recombinant Expression of Human IL-33 Protein and Its Effect on Skin Wound Healing in Diabetic Mice. Bioengineering 2022, 9, 734. https://doi.org/10.3390/bioengineering9120734
Li Y, Lin S, Xiong S, Xie Q. Recombinant Expression of Human IL-33 Protein and Its Effect on Skin Wound Healing in Diabetic Mice. Bioengineering. 2022; 9(12):734. https://doi.org/10.3390/bioengineering9120734
Chicago/Turabian StyleLi, Yunxian, Shixin Lin, Sheng Xiong, and Qiuling Xie. 2022. "Recombinant Expression of Human IL-33 Protein and Its Effect on Skin Wound Healing in Diabetic Mice" Bioengineering 9, no. 12: 734. https://doi.org/10.3390/bioengineering9120734
APA StyleLi, Y., Lin, S., Xiong, S., & Xie, Q. (2022). Recombinant Expression of Human IL-33 Protein and Its Effect on Skin Wound Healing in Diabetic Mice. Bioengineering, 9(12), 734. https://doi.org/10.3390/bioengineering9120734