Wine Traceability with Rare Earth Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection
2.3. Sample Treatment
- Soil samples were treated according to a standardized procedure: soil was dried at 105 °C for 24 h, after which 1 g was sieved (φ 0.2 mm) and extracted with 20 mL of hydrogen peroxide for 20 min and then with 12 mL of aqua regia on a heating plate for 2 h under reflux. The resulting solution was diluted to volume in a 100 mL volumetric flask with high-purity water.
- The grapes were processed as follows: skins were manually separated from the pulp and seeds and transferred to different Pyrex glass containers. All parts were separately subjected to dry ashing in porcelain crucibles in a Pyro 260 microwave ashing system (Milestone, Sorisole, Italy) with the following temperature cycle: 15 min to 150 °C, 60 min to 1000 °C, and 10 min at 1000 °C. The resulting ash was dissolved in 1 mL of concentrated nitric acid and brought to 50 mL to obtain a nitric acid concentration of approximately 1%. All solutions were prepared with high-purity water.
- Musts (100 g) were dried overnight at 105 °C. The dried samples were transferred to porcelain crucibles and subjected to ashing with the following temperature cycle: 50 min to 750 °C, 10 min at 750 °C, 10 min to 900 °C, and 10 min at 900 °C. The resulting ash was dissolved in 1 mL of concentrated nitric acid and brought to 50 mL to obtain a nitric acid concentration of approximately 1%. All solutions were prepared with high-purity water.
- Wine samples, obtained after every passage in the vinification, were treated with microwave ashing in the same condition used for must.
2.4. Vinification Processes
2.5. ICP-MS Analysis
2.6. Analysis of Certified Samples
2.7. Data Analysis
3. Results and Discussion
3.1. Distribution of REEs in Different Parts of the Grapes
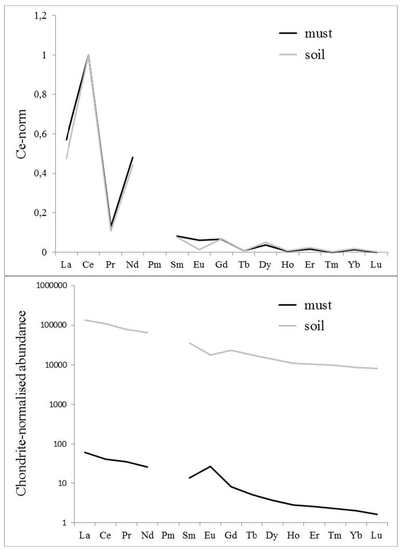
3.2. Comparison of Soil and Must
3.3. Effect of the Vinification Processes
3.4. Effect of Vintage
3.5. Multivariate Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furia, E.; Naccarato, A.; Sindona, G.; Stabile, G.; Tagarelli, A. Multielement fingerprinting as a tool in origin authentication of PGI food products: Tropea Red Onion. J. Agric. Food Chem. 2011, 59, 8450–8457. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkamel, H.; Di Donna, L.; Mazzotti, F.; Naccarato, A.; Sindona, G.; Tagarelli, A.; Taverna, D. Authenticity of PGI “Clementine of Calabria” by multielement fingerprint. J. Agric. Food Chem. 2012, 60, 3717–3726. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, V.; Miazzi, M.M.; Fanelli, V.; Savino, V.; Pollastro, S.; Colucci, F.; Miccolupo, A.; Blanco, A.; Pasqualone, A.; Montemurro, C. An enhanced analytical procedure to discover table grape DNA adulteration in industrial musts. Food Control 2016, 60, 124–130. [Google Scholar] [CrossRef]
- Pasqualone, A.; Alba, V.; Mangini, G.; Blanco, A.; Montemurro, C. Durum wheat cultivar traceability in PDO Altamura bread by analysis of DNA microsatellites. Eur. Food Res. Technol. 2010, 230, 723–729. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Georgiou, C.A. Multi-element and multi-isotope-ratio analysis to determine the geographical origin of foods in the European Union. TRAC Trends Anal. Chem. 2012, 40, 38–51. [Google Scholar] [CrossRef]
- Rossmann, A. Determination of stable isotope ratios in food analysis. Food Rev. Int. 2001, 17, 347–381. [Google Scholar] [CrossRef]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Rare earth elements in biological systems. In Handbook on the Physics and Chemistry of Rare Earths; Schneider, K.A., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; Volume 13, pp. 423–452. ISBN 978-0-444-88547-0. [Google Scholar]
- Tyler, G. Rare earth elements in soil and plant systems: A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Liang, T.; Ding, S.; Song, W.; Chong, Z.; Zhang, C.; Li, H. A review of fractionations of rare earth elements in plants. J. Rare Earth 2008, 26, 7–15. [Google Scholar] [CrossRef]
- Oddone, M.; Aceto, M.; Baldizzone, M.; Musso, D.; Osella, D. Authentication and traceability study of hazelnuts from Piedmont, Italy. J. Agric. Food Chem. 2009, 57, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Musso, D.; Calà, E.; Arieri, F.; Oddone, M. Role of lanthanides in the traceability of the milk production chain. J. Agric. Food Chem. 2017, 65, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Gonzálvez, A.; de la Guardia, M. Mineral Profile. In Food Protected Designation of Origin: Methodologies and Applications; de la Guardia, M., Gonzálvez, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Comprehensive Analytical Chemistry; Volume 60, pp. 51–76. ISBN 978-0-444-59562-1. [Google Scholar]
- Marengo, E.; Aceto, M. Statistical investigation of the differences in the distribution of metals in Nebbiolo-based wines. Food Chem. 2003, 81, 621–630. [Google Scholar] [CrossRef]
- Vaclavik, L.; Lacina, O.; Hajslova, J.; Zweigenbaum, J. The use of high performance liquid chromatography–quadrupole time-of-flight mass spectrometry coupled to advanced data mining and chemometric tools for discrimination and classification of red wines according to their variety. Anal. Chim. Acta 2011, 685, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Marengo, E.; Aceto, M.; Maurino, V. Classification of Nebbiolo-based wines from Piedmont (Italy) by means of solid-phase microextraction-gas chromatography-mass spectrometry of volatile compounds. J. Chromatogr. A 2002, 943, 123–137. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Vasconcelos, M.T.S.D. Does the winemaking process influence the wine 87Sr/86Sr? A case study. Food Chem. 2004, 85, 7–12. [Google Scholar] [CrossRef]
- Marchionni, S.; Braschi, E.; Tommasini, S.; Bollati, A.; Cifelli, F.; Mulinacci, N.; Conticelli, S. High-Precision Sr-87/Sr-86 analyses in wines and their use as a geological fingerprint for tracing geographic provenance. J. Agric. Food Chem. 2013, 61, 6822–6831. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Tufariello, M.; Francioso, L.; Montagna, G.; Casino, F.; Leone, A.; Siciliano, P. Aroma analysis by GC/MS and electronic nose dedicated to Negroamaro and Primitivo typical Italian Apulian wines. Sens. Actuators B Chem. 2013, 179, 259–269. [Google Scholar] [CrossRef]
- Jaitz, L.; Siegl, K.; Eder, R.; Rak, G.; Abranko, L.; Koellensperger, G.; Hann, S. LC–MS/MS analysis of phenols for classification of red wine according to geographic origin, grape variety and vintage. Food Chem. 2010, 122, 366–372. [Google Scholar] [CrossRef]
- Aceto, M. The use of ICP-MS in food traceability. In Advances in Food Traceability Techniques and Technologies: Improving Quality throughout the Food Chain; Espiñeira, M., Santaclara, F.J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 137–164. ISBN 978-00-8100-321-3. [Google Scholar]
- Hopfer, H.; Nelson, J.; Collins, T.S.; Heymann, H.; Ebeler, S.E. The combined impact of vineyard origin and processing winery on the elemental profile of red wines. Food Chem. 2015, 172, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.F.; Longerich, H.P.; Greenough, J.D. Multielement analysis of Canadian wines by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and multivariate statistics. J. Agric. Food Chem. 2003, 51, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Baldizzone, M.; Oddone, M. Keeping the track of quality: Authentication and traceability studies on wine. In Red Wine and Health; O’Byrne, P., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 429–466. ISBN 978-1606927182. [Google Scholar]
- Aceto, M.; Robotti, E.; Oddone, M.; Baldizzone, M.; Bonifacino, G.; Bezzo, G.; Di Stefano, R.; Gosetti, F.; Mazzucco, E.; Manfredi, M.; et al. A traceability study on the Moscato wine chain. Food Chem. 2013, 138, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Di Paola-Naranjo, R.D.; Baroni, M.V.; Podio, N.S.; Rubinstein, H.R.; Fabani, M.P.; Badini, R.G.; Inga, M.; Ostera, H.A.; Cagnoni, M.; Gallegos, H.; et al. Fingerprints for main varieties of Argentinean wines: Terroir differentiation by inorganic, organic, and stable isotopic analyses coupled to chemometrics. J. Agric. Food Chem. 2011, 59, 7854–7865. [Google Scholar] [CrossRef] [PubMed]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- May, T.W.; Wiedmeyer, R.H. A table of polyatomic interferences in ICP-MS. Atom. Spectrosc. 1998, 19, 150–155. [Google Scholar]
- Censi, P.; Saiano, F.; Pisciotta, A.; Tuzzolino, N. Geochemical behaviour of rare earths in Vitis vinifera grafted onto different rootstocks and growing on several soils. Sci. Total Environ. 2014, 473–474, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Tutone, L.; Saiano, F. Distribution of YLOID in soil-grapevine system (Vitis vinifera L.) as tool for geographical characterization of agro-food products. A two years case study on different grafting combinations. Food Chem. 2017, 221, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Pepi, S.; Sansone, L.; Chicca, M.; Marrocchino, E.; Vaccaro, C. Distribution of rare earth elements in soil and grape berries of Vitis vinifera cv. “Glera”. Environ. Monit. Assess. 2016, 188, 477. [Google Scholar] [CrossRef] [PubMed]






| Element | Certified Values (µg/Kg) | Uncertainty | Found (µg/Kg) | s.d. * |
|---|---|---|---|---|
| La | 80 | 6 | 76.12 | 1.87 |
| Ce | 89 | 7 | 106.41 | 4.30 |
| Pr | 12.3 | 1.1 | 13.21 | 0.36 |
| Nd | 54 | 4 | 52.54 | 1.70 |
| Sm | 11.2 | 0.8 | 11.02 | 0.89 |
| Eu | 2.79 | 0.16 | 3.14 | 0.13 |
| Gd | 13 | 0.6 | 12.93 | 1.15 |
| Tb | 1.62 | 0.12 | 1.66 | 0.18 |
| Dy | 8.9 | 0.6 | 8.39 | 0.60 |
| Ho | 1.8 1 | 0.60 1 | 1.62 | 0.20 |
| Er | 4.5 | 0.5 | 4.27 | 0.31 |
| Tm | 0.48 | 0.08 | 0.60 | 0.02 |
| Yb | 2.8 1 | 0.5 1 | 3.05 | 0.47 |
| Lu | 0.389 | 0.024 | 0.59 | 0.04 |
| Element | Certified Values (µg/Kg) | Uncertainty | Found (µg/Kg) | s.d. |
|---|---|---|---|---|
| La | 487 | 20 | 474.1 | 4.3 |
| Ce | 990 | 40 | 959 | 134 |
| Pr | 121 | 6 | 115.2 | 11.1 |
| Nd | 473 | 15 | 483.1 | 14.5 |
| Sm | 94 | 7 | 96.10 | 0.53 |
| Eu | 23.2 | 1.5 | 76.12 | 0.32 |
| Gd | 98 | 8 | 105.61 | 6.37 |
| Tb | 14 | 1.1 | 13.24 | 2.12 |
| Dy | 79 | 7 | 80.22 | 4.91 |
| Ho | 15.8 | 1.8 | 16.51 | 2.98 |
| Er | 44 | 2.8 | 46.11 | 1.62 |
| Tm | 5.7 | 0.7 | 5.23 | 1.13 |
| Yb | 40 | 4 | 43.32 | 6.14 |
| Lu | 6.3 | 0.5 | 7.11 | 0.93 |
| Element | Certified Values (mg/Kg) | Uncertainty | Found (mg/Kg) | s.d. |
|---|---|---|---|---|
| La | 29.7 | 4.8 | 27.11 | 0.96 |
| Ce | 58 | 8 | 56.82 | 1.64 |
| Pr | 7.3 1 | 7.51 | 0.21 | |
| Nd | 26.4 | 2.9 | 26.14 | 0.81 |
| Sm | 6.1 1 | 5.22 | 0.189 | |
| Eu | 1.5 1 | 0.98 | 0.04 | |
| Gd | 5.8 1 | 4.82 | 0.18 | |
| Tb | 0.9 1 | 0.68 | 0.03 | |
| Dy | 5.4 1 | 3.52 | 0.15 | |
| Ho | 1.1 1 | 0.63 | 0.03 | |
| Er | 3.30 1 | 1.86 | 0.03 | |
| Tm | 0.5 1 | 0.23 | 0.02 | |
| Yb | 2.64 | 0.51 | 1.31 | 0.04 |
| Lu | 2 | 0.15 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aceto, M.; Bonello, F.; Musso, D.; Tsolakis, C.; Cassino, C.; Osella, D. Wine Traceability with Rare Earth Elements. Beverages 2018, 4, 23. https://doi.org/10.3390/beverages4010023
Aceto M, Bonello F, Musso D, Tsolakis C, Cassino C, Osella D. Wine Traceability with Rare Earth Elements. Beverages. 2018; 4(1):23. https://doi.org/10.3390/beverages4010023
Chicago/Turabian StyleAceto, Maurizio, Federica Bonello, Davide Musso, Christos Tsolakis, Claudio Cassino, and Domenico Osella. 2018. "Wine Traceability with Rare Earth Elements" Beverages 4, no. 1: 23. https://doi.org/10.3390/beverages4010023
APA StyleAceto, M., Bonello, F., Musso, D., Tsolakis, C., Cassino, C., & Osella, D. (2018). Wine Traceability with Rare Earth Elements. Beverages, 4(1), 23. https://doi.org/10.3390/beverages4010023