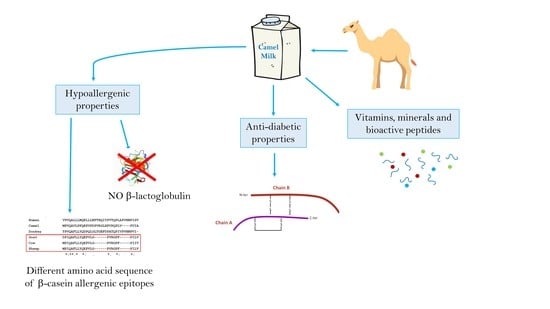
Nutraceutical and Functional Properties of Camelids’ Milk
Abstract
:1. Introduction
2. Dromedary and Bactrian Camel Milk
3. Protein Fractions in Camel Milk
4. Lipid Fractions in Camel Milk
5. Minerals and Vitamins in Camel Milk
6. Therapeutic Properties of Camel Milk
6.1. Hypoallergenic Properties of Camel Milk
6.2. Camel Milk for Autoimmune Disease
6.3. Camel Milk in the Treatment of Autism
6.4. Camel Milk in the Treatment of Tuberculosis and MDR Tuberculosis
6.5. Camel Milk in the Treatment of Diabetes
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, C.M.; Ramachandra, C.T.; Kumar, G.M. A Comprehensive Review on Composition of Donkey Milk in Comparison to Human, Cow, Buffalo, Sheep, Goat, Camel and Horse Milk. Mysore J. Agric. Sci. 2020, 54, 42–50. [Google Scholar]
- Polidori, P.; Vincenzetti, S. Use of Donkey Milk in Children with Cow’s Milk Protein Allergy. Foods 2013, 2, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restani, P.; Ballabio, C.; Di Lorenzo, C.; Tripodi, S.; Fiocchi, A. Molecular aspects of milk allergens and their role in clinical events. Anal. Bioanal. Chem. 2009, 395, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Xu, S.; Villalobos-Santeli, J.A.; Huang, J.-Y. Fouling characterization of camel milk with comparison to bovine milk. J. Food Eng. 2020, 285, 110085. [Google Scholar] [CrossRef]
- Izadi, A.; Rahbarimanesh, A.A.; Mojtahedi, Y.; Mojtahedi, S.Y. Prevalence of enterovirus meningitis in children: Report from a tertiary center. Maedica—J. Clin. Med. 2018, 13, 213–216. [Google Scholar]
- El-Agamy, E.I. The challenge of cow milk protein allergy. Small Rumin. Res. 2007, 68, 64–72. [Google Scholar] [CrossRef]
- Iacono, G.; Carroccio, A.; Cavataio, F.; Montalto, G.; Soresi, M.; Balsamo, V. Use of ass’s milk in multiple food allergy. J. Pediatr. Gastroent. Nutr. 1992, 14, 177–181. [Google Scholar] [CrossRef]
- Monti, G.; Viola, S.; Baro, C.; Cresi, F.; Tovao, P.A.; Moro, G.; Ferrero, M.P.; Conti, A.; Bertino, E. Tolerability of donkey’s milk in 92 highly problematic cow’s milk allergic children. J. Biol. Regul. Homeost. Agents 2012, 26, 75–82. [Google Scholar]
- Izadi, A.; Khedmat, L.; Mojtahedi, S.Y. Nutritional and therapeutic perspectives of camel milk and its protein hydrolysates: A review on versatile biofunctional properties. J. Funct. Foods 2019, 60, 103441. [Google Scholar] [CrossRef]
- Shabo, Y.; Barzel, R.; Margoulis, M.; Yagil, R. Camel milk for food allergies in children. Israel Med. Assoc. J. 2005, 7, 796–798. [Google Scholar]
- Mojtahedi, S.Y.; Izadi, A.; Seirafi, G.; Khedmat, L.; Tavakolizadeh, R. Risk factors associated with neonatal jaundice: A cross-sectional study from Iran. Macedon. J. Med. Sci. 2018, 6, 1387–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraz, A.; Waheed, A.; Tauqir, N.A.; Mirza, R.H.; Ishaq, H.M.; Nabeel, M.S. Characteristics and composition of camel (Camelus dromedarius) milk: The white gold of desert. Adv. Anim. Vet. Sci. 2020, 8, 766–770. [Google Scholar] [CrossRef]
- El-Agamy, S.I.; Ruppanner, R.; Ismail, A.; Champagne, C.P.; Assaf, R.J. Antibacterial and Antiviral activity of camel milk protective proteins. J. Dairy Res. 1992, 59, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kaskous, S. Importance of camel milk for human health. Emir. J. Food Agric. 2016, 28, 158–163. [Google Scholar] [CrossRef]
- Ayyash, M.; Abdalla, A.; Alhammadi, A.; Ranadheera, C.S.; Affan Baig, M.; Al-Ramadi, B.; Chen, G.; Kamal-Eldin, A.; Huppertz, T. Probiotic survival, biological functionality and untargeted metabolomics of the bioaccessible compounds in fermented camel and bovine milk after in vitro digestion. Food Chem. 2021, 363, 130243. [Google Scholar] [CrossRef]
- Faccia, M.; D’Alessandro, A.G.; Summer, A.; Hailu, Y. Milk Products from Minor Dairy Species: A Review. Animals 2020, 10, 1260. [Google Scholar] [CrossRef]
- Fantuz, F.; Salimei, E.; Papademas, P. Macro-and Micronutrients in Non-cow Milk and Products and Their Impact on Human Health. In Non-Bovine Milk and Milk Products; Tsakalidou, E., Papadimitriou, K., Eds.; Academic Press: London, UK, 2016; pp. 209–261. [Google Scholar]
- Gerosa, S.; Skoet, J. Milk availability: Current production and demand and medium-term outlook. In Milk and Dairy Products in Human Nutrition; FAO: Rome, Italy, 2013; pp. 11–40. [Google Scholar]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caufield, L.E.; de Oris, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Molgaard, C.; Girma, T.; Friis, H. Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food Nutr. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H. Global dietary patterns and diets in childhood: Implications for health outcomes. Ann. Nutr. Metabol. 2012, 61 (Suppl. 1), 29–37. [Google Scholar] [CrossRef]
- Almeida, R.R. Camel Milk: Characteristics and Perspectives for Use in Clinical Practice. Rev. Chil. Nutr. 2011, 38, 211–218. [Google Scholar]
- Farah, Z. Composition and characteristics of camel milk. J. Dairy Res. 1993, 60, 603–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraz, A. Composition of Camel Milk: A Blessing for Health. Ann. Public Health Epidemiol. 2020, 1, 1–4. [Google Scholar]
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2012, 92, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Polidori, P.; Cammertoni, N.; Santini, G.; Klimanova, Y.; Zhang, J.J.; Vincenzetti, S. Nutritional Properties of Camelids and Equids Fresh and Fermented Milk. Dairy 2021, 2, 288–302. [Google Scholar] [CrossRef]
- Singh, R.; Mal, G.; Kumar, D.; Patil, N.V.; Pathac, K.M.L. Camel Milk: An Important Natural Adjuvant. Agric. Res. 2017, 6, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Faye, B.; Konuspayeva, G. The sustainability challenge of the dairy sector- the growing importance of the non-cattle milk production worldwide. Int. Dairy J. 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Gorban, M.A.S.; Izzeldin, O.M. Mineral content of camel milk and colostrum. J. Dairy Res. 1997, 64, 471–474. [Google Scholar] [CrossRef]
- Ayadi, M.; Hammadi, M.; Khorchani, T.; Barmat, A.; Atigui, M.; Caja, G. Effects of milking interval and cisternal udder evaluation in Tunisian Maghrebi dairy dromedaries (Camelus dromedarius L.). J. Dairy Sci. 2009, 92, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Zibaee, S.; Hosseini, M.S.; Yousefi, M.; Taghipour, A.; Kiani, M.A.; Noras, M.R. Nutritional and Therapeutic Char acteristics of Camel Milk in Children: A Systematic Review. Electr. Phys. 2015, 7, 1523–1528. [Google Scholar]
- Mehta, B.M.; Aparnathi, K.; Yoganandi, J.; Wadhwani, K.; Darji, V. Comparison of physico-chemical properties of camel milk with cow milk and buffalo milk. J. Camel Pract. Res. 2014, 21, 253–258. [Google Scholar]
- Yadav, A.K.; Kumar, R.; Priyadarshini, L.; Singh, J. Composition and medicinal properties of camel milk: A Review. Asian J. Dairy Food Res. 2015, 34, 83–91. [Google Scholar] [CrossRef]
- Idrees, E.M.; Ishag, I.A.; Eisa, M.O. Factors Affecting Chemical Properties of Camel Milk. Sci. Agric. 2016, 16, 49–53. [Google Scholar]
- Saleh, S.K.; Al-Ramadhan, G.; Faye, B. Monitoring of monthly SCC in she-camel in relation to milking practice, udder status and microbiological contamination of milk. Emir. J. Food Agric. 2013, 25, 403–408. [Google Scholar]
- Mati, A.; Senoussi-Ghezali, C.; Zennia, S.S.A.; Almi-Sebbane, D.; El-Hatmi, H.; Girardet, J.M. Dromedary camel milk proteins, a source of peptides having biological activities—A review. Int. Dairy J. 2017, 73, 25–37. [Google Scholar] [CrossRef]
- Kappeler, S.; Farah, Z.; Puhan, Z. Sequence analysis of Camelus dromedarius milk caseins. J. Dairy Res. 1998, 65, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Vincenzetti, S.; Pucciarelli, S.; Polzonetti, V.; Polidori, P. Role of Proteins and of Some Bioactive Peptides on the Nutritional Quality of Donkey Milk and Their Impact on Human Health. Beverages 2017, 3, 34. [Google Scholar] [CrossRef]
- Sharma, P.; Dube, D.; Singh, A.; Mishra, B.; Singh, N.; Sinha, M.; Dey, S.; Kaur, P.; Mitra, D.K.; Sharma, S.; et al. Structural Basis of Recognition of Pathogen-associated Molecular Patterns and Inhibition of Proinflammatory Cytokines by Camel Peptidoglycan Recognition Protein. J. Biol. Chem. 2011, 286, 16208–16217. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk from Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Barłowska, J.; Szwajkowska, M.; Litwinczuk, Z.; Krόl, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Comprehen. Rev. Food Sci. Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Navrátilová, P.; Borkovcová, I.; Kaniová, L.; Dluhošová, S.; Zachovalová, H. The content of selected vitamins and iodine in mare’s milk. Acta Vet. Brno 2019, 88, 473–480. [Google Scholar] [CrossRef]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary Intake of Vitamin D from Dairy Products Reduces the Risk of Osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, M.S.Y.; Gammoh, S.I.; Robinson, R.K. Seasonal variations in the chemical composition of camel milk in Jordan. J. Dairy Res. 2008, 75, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, S.A.; Majid, A.M.A.; Nikhala, A.M. Camels (Camelus dromedarius) under pastoral systems in North Kordofan Sudan: Seasonal and parity effects on milk composition. J. Camelid Sci. 2008, 1, 32–36. [Google Scholar]
- Restani, P.; Gaiaschi, A.; Plebani, A.; Beretta, B.; Cavagni, G.; Fiocchi, A.; Poiesi, C.; Velona, A.; Ugazio, G.; Galli, C.L. Cross-reactivity between milk proteins from different animal species. Clin. Exp. Allergy 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Ehlayel, M.; Bener, A.; Abu Hazeima, K.; Al-Mesaifri, F. Camel milk is a safer choice than goat milk for feeding children with cow milk allergy. ISRN Allergy 2011, 29, 391641. [Google Scholar] [CrossRef] [Green Version]
- Navarrete-Rodríguez, E.M.; Ríos-Villalobos, L.A.; Alcocer-Arreguín, C.R.; Del-Rio-Navarro, B.E.; Del Rio-Chivardi, J.M.; Saucedo-Ramírez, O.J.; Sienra-Monge, J.J.L.; Frias, R.V. Cross-over clinical trial for evaluating the safety of camel’s milk intake in patients who are allergic to cow’s milk protein. Allergol. Immunopathol. 2017, 4, 149–154. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Hansen, E.B.; Ballegaard, A.R.; Sancho, A.I.; Bøgh, K.L. Comparison of the Allergenicity and Immunogenicity of Camel and Cow’s Milk-A Study in Brown Norway Rats. Nutrients 2018, 10, 1903. [Google Scholar] [CrossRef] [Green Version]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Vila, L.; Beyer, K.; Sampson, H.A. Identification of IgE and IgG binding epitopes on beta- and kappa-casein in cow’s milk allergic patients. Clin. Exp. Allergy 2001, 31, 1256–1262. [Google Scholar] [CrossRef]
- Mal, G.; Suchitra, S.D.; Sahani, M.S. Changes in chemical and macrominerals content of dromedary milk during lactation. J. Camel Pract. Res. 2007, 14, 195–197. [Google Scholar]
- Shabo, Y.; Yagil, R. Etiology of autism and camel milk as therapy. Int. J. Disab. Hum. Dev. 2005, 4, 67–70. [Google Scholar] [CrossRef]
- Mal, G.; Suchitra, S.D.; Jain, V.K.; Sahani, M.S. Therapeutic value of camel milk as a nutritional supplement for multiple drug resistant (MDR) tuberculosis patients. Israel J. Vet. Med. 2006, 61, 88–94. [Google Scholar]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, M.A.; Palakkott, A.R.; Ashraf, A.; Iratni, R. The molecular basis of the anti-diabetic properties of camel milk. Diabetes Res. Clin. Pract. 2018, 146, 305–312. [Google Scholar] [CrossRef]
- Malik, A.; Al-Senaidy, A.; Skrzypczak-Jankun, E.; Jankun, J. A study of the anti-diabetic agents of camel milk. Int. J. Mol. Med. 2012, 30, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Shori, A.B. Camel milk as a potential therapy for controlling diabetes and its complications: A review of in vivo studies. J. Food Drug Anal. 2015, 23, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.P.; Kochar, D.K.; Sahani, M.S.; Tuteja, F.C.; Ghrui, S.K. Hypoglycaemic activity of camel milk in streptozotocin induced diabetic rats. Int. J. Diab. Dev. Count 2004, 24, 47–49. [Google Scholar]
- Breitling, L. Insulin and antidiabetic activity of camel milk. J. Camel Pract. Res. 2002, 9, 43–45. [Google Scholar]
- Graves, D.T.; Kayal, R.A. Diabetic complications and dysregulated innate immunity. Front. Biosci. 2008, 13, 1227–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Shamsia, S.M. Nutritional and therapeutic properties of camel and human milks. Int. J. Genet. Mol. Biol. 2009, 1, 52–58. [Google Scholar]
- Faye, B. Camel farming sustainability: The challenges of the camel farming system in the XXI century. J. Sustain. Devel. 2013, 6, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Abu-Lehiya, I.H. Composition of camel milk. Milchwiss 1987, 42, 368–371. [Google Scholar]
- Berhe, T.; Vogensen, F.K.; Ipsen, R.; Seifu, E.; Kurtu, M.Y. Traditional fermented dairy products of Ethiopia: A review. East Afr. J. Sci. 2017, 11, 73–80. [Google Scholar]
- Stahl, T.; Sallman, H.I.; Duehlmeier, R.R.; Wernery, U. Selected vitamins and fatty acid patterns in dromedary milk and colostrum. J. Camel Prac. Res. 2006, 13, 53–57. [Google Scholar]
- El Zeini, H.M. Microstructure, rheological and geometrical properties of fat globules of milk from different animal species. Pol. J. Food Nutr. Sci. 2006, 15, 147–153. [Google Scholar]



| Mammal | Energy (kcal/100 g) | Fat (g/100 g) | Protein (g/100 g) | Lactose (g/100 g) |
|---|---|---|---|---|
| Human | 64.2 | 3.5 | 1.2 | 6.4 |
| Donkey | 41.8 | 0.7 | 1.6 | 6.6 |
| Cow | 76.2 | 3.8 | 3.4 | 4.8 |
| Sheep | 115.7 | 7.0 | 5.7 | 4.7 |
| Goat | 74.5 | 4.1 | 3.3 | 4.5 |
| Dromedary | 66.1 | 3.1 | 3.5 | 4.4 |
| Bactrian camel | 88.9 | 5.3 | 3.9 | 4.5 |
| Protein Fraction | Cow | Goat | Sheep | Camel | Donkey | Human |
|---|---|---|---|---|---|---|
| Total caseins | 24.6–28 | 23.3–46.3 | 41.8–52.6 | 22.1–26.0 | 6.4–10.3 | 2.4–4.2 |
| Total whey proteins | 5.5–7.0 | 3.7–7.0 | 10.2–16.1 | 5.9–8.1 | 4.9–8.0 | 6.2–8.3 |
| Caseins/whey proteins ratio | 82:18 | 78:22 | 76:24 | 73.2:76.2 | 56:44 | 29.7:33.7 |
| αS1-Casein | 8.0–10.7 | 0–13.0 | 2.4–22.1 | 4.9–5.7 | Present | 0.77 |
| αS2-Casein | 2.8–3.4 | 2.3–11.6 | 6.0 | 2.1–2.5 | Present | Absent |
| β-Casein | 8.6–9.3 | 0–29.6 | 15.6–39.6 | 14.4–16.9 | Present | 3.87 |
| k-Casein | 2.3–3.3 | 2.8–13.4 | 3.2–12.2 | 0.8–0.9 | Present | 0.14 |
| β-Lactoglobulin | 3.2–3.3 | 1.5–5.0 | 6.5–13.5 | Absent | 3.3 | Absent |
| α-Lactalbumin | 1.2–1.3 | 0.7–2.3 | 1–1.9 | 0.8–3.5 | 1.9 | 1.9–3.4 |
| Amino Acid | Cow | Sheep | Goat | Camel | Donkey | Human |
|---|---|---|---|---|---|---|
| Aspartic acid | 7.8 | n.d. | 7.4 | 6.9 | 8.9 | 8.3 |
| Threonine | 4.5 | 4.2–4.4 | 5.7 | 4.1 | 3.6 | 4.6 |
| Serine | 4.8 | n.d. | 5.2 | 4.3 | 6.2 | 5.1 |
| Glutamic acid | 23.2 | n.d. | 19.3 | 18.1 | 22.8 | 17.8 |
| Proline | 9.6 | n.d. | 14.6 | 12.0 | 8.8 | 8.6 |
| Cysteine | 0.6 | 0.8–0.9 | 0.6 | 1.9 | 0.4 | 1.7 |
| Glycine | 1.8 | n.d. | 2.1 | 2.1 | 1.2 | 2.6 |
| Alanine | 3.0 | n.d. | 3.6 | 2.1 | 3.5 | 4.0 |
| Valine | 4.8 | 6.2–6.4 | 5.7 | 4.1 | 6.5 | 6.0 |
| Methionine | 1.8 | 2.7 | 3.5 | 2.0 | 1.8 | 1.8 |
| Isoleucine | 4.2 | 4.6 | 7.1 | 4.9 | 5.5 | 5.8 |
| Leucine | 8.7 | 9.7–9.9 | 8.2 | 6.1 | 8.6 | 10.1 |
| Tyrosine | 4.5 | 3.7–3.8 | 4.8 | 3.1 | 3.7 | 4.7 |
| Phenylalanine | 4.8 | 4.2–4.3 | 6.0 | 4.0 | 4.3 | 4.4 |
| Histidine | 3.0 | n.d. | 5.0 | 2.1 | 2.3 | 2.3 |
| Lysine | 8.1 | 7.7–7.8 | 8.2 | 4.0 | 7.3 | 6.2 |
| Arginine | 3.3 | n.d. | 2.9 | 2.0 | 4.6 | 4.0 |
| Tryptophan | 1.5 | n.d. | n.d. | n.d. | n.d. | 1.8 |
| Limiting amino acid | Cysteine Methionine | ---- | ---- | Lysine | Cysteine Methionine | ---- |
| Fatty Acids | Cow | Sheep | Goat | Camel | Donkey | Human |
|---|---|---|---|---|---|---|
| SFA (%) | 55.7–72.8 | 57.5–74.6 | 59.9–73.7 | 47.0–69.9 | 46.7–67.7 | 39.4–45.0 |
| MUFA (%) | 22.7–30.3 | 23.0–39.1 | 21.8–35.9 | 28.1–31.1 | 15.3–35.0 | 33.2–45.1 |
| PUFA (%) | 2.4–6.3 | 2.5–7.3 | 2.6–5.6 | 1.8–11.1 | 14.2–30.5 | 8.1–19.1 |
| ω6/ω3 | 2.1–3.7 | 1.0–3.8 | 4 | n.d. | 0.9–6.1 | 7.4–8.1 |
| CLA (%) | 0.2–2.4 | 0.6–1.1 | 0.3–1.2 | 0.4–1.0 | n.d. | 0.2–1.1 |
| Cholesterol (mg/100 mL milk) | 13.1–31.4 | 14.0–29.0 | 10.7–18.1 | 31.3–37.1 | n.d. | 14.0–20.0 |
| Macroelements (mg/100 g) | Cow | Sheep | Goat | Mare | Camel | Human |
|---|---|---|---|---|---|---|
| Calcium | 122.0 | 195–200 | 132–134 | 132.7 | 114–116 | 33.0 |
| Phosphorus | 119.0 | 124–158 | 97.7–121 | 88.4 | 87.4 | 43.0 |
| Potassium | 152.0 | 136–140 | 152–181 | 66.5 | 144–156 | 55.0 |
| Magnesium | 12.0 | 18–21 | 15.8–16.0 | 10.2 | 10.5–12.3 | 4.0 |
| Sodium | 58.0 | 44–58 | 41–59.4 | 19.8 | 59.0 | 15.0 |
| Microelements (µg/100 g) | ||||||
| Zinc | 530.0 | 520–747 | 56–370 | 270.0 | 530–590 | 380.0 |
| Iron | 80.0 | 72–122 | 7.0–60 | 37.0 | 230–290 | 200.0 |
| Copper | 60.0 | 40–68 | 5.0–80.0 | 64.0 | 140.0 | 60.0 |
| Manganese | 20.0 | 5.3–9.0 | 3.2–6.53 | n.d. | 80.0 | 70.0 |
| Iodine | 2.1 | 10.4 | 2.2 | n.d. | n.d. | 7.0 |
| Selenium | 0.96 | 3.1 | 1.33 | n.d. | n.d. | 1.52 |
| Vitamin | Goat | Sheep | Cow | Camel | Human |
|---|---|---|---|---|---|
| Vitamin A (µg/100 g) | 185 | 146 | 126 | 26.7 | 190 |
| Vitamin D (µg/100 g) | 2.3 | 1.18 | 2.0 | 0.3 | 1.4 |
| Vitamin B1 (mg/100 g) | 0.07 | 0.08 | 0.05 | 0.05 | 0.02 |
| Vitamin B2 (mg/100 g) | 0.21 | 0.38 | 0.16 | 0.17 | 0.02 |
| Vitamin B3 (mg/100 g) | 0.27 | 0.42 | 0.08 | 0.77 | 0.17 |
| Vitamin B5 (mg/100 g) | 0.31 | 0.41 | 0.32 | 0.37 | 0.20 |
| Vitamin B6 (mg/100 g) | 0.05 | 0.08 | 0.04 | 0.55 | 0.01 |
| Vitamin B8 (µg/100 g) | 1 | 5 | 5 | 87 | 5.5 |
| Vitamin B9 (µg/100 g) | 1.5 | 0.93 | 2 | n.d. | 0.4 |
| Vitamin B12 (µg/100 g) | 0.07 | 0.71 | 0.36 | 85 | 0.03 |
| Vitamin C (mg/100 g) | 1.29 | 4.16 | 0.94 | 33 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenzetti, S.; Cammertoni, N.; Rapaccetti, R.; Santini, G.; Klimanova, Y.; Zhang, J.-J.; Polidori, P. Nutraceutical and Functional Properties of Camelids’ Milk. Beverages 2022, 8, 12. https://doi.org/10.3390/beverages8010012
Vincenzetti S, Cammertoni N, Rapaccetti R, Santini G, Klimanova Y, Zhang J-J, Polidori P. Nutraceutical and Functional Properties of Camelids’ Milk. Beverages. 2022; 8(1):12. https://doi.org/10.3390/beverages8010012
Chicago/Turabian StyleVincenzetti, Silvia, Natalina Cammertoni, Roberta Rapaccetti, Giuseppe Santini, Yulia Klimanova, Jing-Jing Zhang, and Paolo Polidori. 2022. "Nutraceutical and Functional Properties of Camelids’ Milk" Beverages 8, no. 1: 12. https://doi.org/10.3390/beverages8010012
APA StyleVincenzetti, S., Cammertoni, N., Rapaccetti, R., Santini, G., Klimanova, Y., Zhang, J.-J., & Polidori, P. (2022). Nutraceutical and Functional Properties of Camelids’ Milk. Beverages, 8(1), 12. https://doi.org/10.3390/beverages8010012