Phenolic Compounds Stability of Grumixama (Eugenia brasiliensis) Juice during Processing and Storage
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
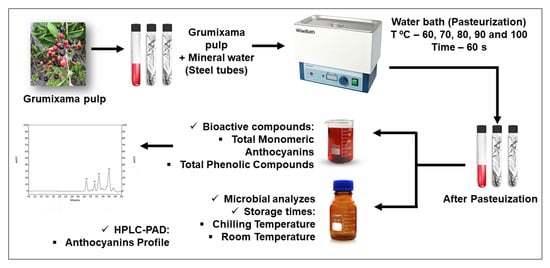
2.2. Juice Processing
2.3. Pasteurization Process
2.4. Pasteurized Juice Stability during Storage
2.5. Physicochemical Properties and Enzymatic Activity
2.6. Total Monomeric Anthocyanins and Total Phenolic Compounds
2.7. HPLC-DAD Analysis of Anthocyanins
2.8. Microbial Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Enzymatic Inactivation and Characterization of Grumixama Juice
3.2. Pasteurization Process
3.2.1. Individual Anthocyanins by HPLC-DAD
3.2.2. Multivariate Analysis for Pasteurization Processes
3.2.3. Effectiveness of the Selected Binomial Time/Temperature
3.2.4. Juice Storage under Different Conditions
3.2.5. Multivariate Analysis for Juice Storage
3.2.6. Microbiological Evaluation of Pasteurized Juice during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant properties and phenolic compounds of vitamin C-rich juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, G.A.; Carvalho, A.V.; Faria, L.J.G.; Chisté, R.C.; Martins, L.H.S.; Lopes, A.S. Effects of thermal pasteurization on jambolan tropical juice bioactive compounds. Br. Food J. 2019, 121, 11. [Google Scholar] [CrossRef]
- Tobar-Bolaños, G.; Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Blueberry juice: Bioactive compounds, health impact, and concentration technologies—A review. J. Food Sci. 2021, 86, 5062–5077. [Google Scholar] [CrossRef]
- Gunathilake, K.D. Emerging technologies for the enhancement of bioactives concentration in functional beverages. In Biotechnological Progress and Beverage Consumption; Grumezescu, A., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–71. [Google Scholar]
- Catalkaya, G.; Guldikenb, B.; Capanoglu, E. Encapsulation of anthocyanin-rich extract from black chokeberry (Aronia melanocarpa) pomace by spray drying using different coating materials. Food Funct. 2022, 13, 11579–11591. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Vega, J.M.; Rios, J.J.; Fett, R.; Troncoso, A.M.; Asuero, A.G. Characterization of anthocyanins from the fruits of baguaçu (Eugenia umbelliflora Berg). J. Agric. Food Chem. 2003, 51, 5450–5454. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.C.H.; Limberger, R.P.; Henriques, A.T.; Moreno, P.R.H. Essential oils from leaves of two Eugenia brasiliensis specimens from southeastern Brazil. J. Essent. Oil Res. 2005, 17, 499–500. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Yang, H.; Jiang, B.; Basile, M.J.; Kennelly, E.J. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008, 109, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Hendrickx, M. Comparing the impact of high pressure. pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Wurlitzer, N.J.; Dionísio, A.P.; Lima, J.R.; Garruti, D.S.; Araújo, I.M.S.; Rocha, R.F.J.; Maia, J.L. Tropical fruit juice: Effect of thermal treatment and storage time on sensory and functional properties. J. Food Sci. Technol. 2019, 56, 5184–5193. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, J.; Li, M.; Li, C. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT—Food Sci. Technol. 2020, 128, 109448. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Oktay, M.; Küfrevioglu, I.; Kocacaliskani, I.; Sakiroglu, H. Polyphenoloxidase from Amasya apple. J. Food Sci. 1995, 60, 494–505. [Google Scholar] [CrossRef]
- Khan, A.A.; Robinson, D.S. Hydrogen donor specificity of mango isoperoxidases. Food Chem. 1994, 49, 407–410. [Google Scholar] [CrossRef]
- Chisté, R.C.; Lopes, A.S.; De Faria, L.J.G. Thermal and light degradation kinetics of anthocyanin extracts from mangosteen peel (Garcinia mangostana L.). Int. J. Food Sci. Technol. 2010, 45, 1902–1908. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Pereira, G.A.; Arruda, H.S.; Pastore, G.M. Modification and validation of Folin-Ciocalteu assay for faster and safer analysis of total phenolic content in food samples. Braz. J. Food Res. 2018, 9, 125–140. [Google Scholar] [CrossRef]
- Flores, G.; Dastmalchi, K.; Paulino, S.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D’armiento, J.M.; Kennelly, E.J. Anthocyanins from Eugenia brasilienis edible fruits as potential therapeutics for COPD treatment. Food Chem. 2012, 134, 1256–1262. [Google Scholar] [CrossRef]
- Qi, Q.; Chuc, M.; Yua, X.; Xie, Y.; Li, Y.; Dua, Y.; Liu, X.; Zhanga, Z.; Shid, J.; Yan, N. Anthocyanins and proanthocyanins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2022, 39, 4581–4609. [Google Scholar] [CrossRef]
- Holzwarth, M.; Wittig, J.; Carle, R.; Kammerer, D.R. Influence of putative polyphenoloxidase (PPO) inhibitors on strawberry (Fragaria x ananassa Duch.) PPO, anthocyanin and color stability of stored purées. LWT—Food Sci. Technol. 2012, 52, 116–122. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Shi, S.; Li, M.; Jin, L.; Qin, G.; Gao, Y.; Ji, J.; Hao, L. Antioxidant properties of anthocyanin revealed through the hydrogen atom transfer: Combined effects of temperature and pH. Mol. Phys. 2021, 119, e1936246. [Google Scholar] [CrossRef]
- Ursu, M.S.; Aprodu, I.; Milea, S.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef]
- Modesto Junior, E.N.; Martins, M.G.; Pereira, G.A.; Chisté, R.C.; Pena, R.S. Stability kinetics of anthocyanins of Grumixama berries (Eugenia brasiliensis Lam.) during thermal and light treatments. Foods 2023, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Qin, Y.; Mao, Y.; Zhang, B.; Deng, Z. Effects of heat, ultrasound, and microwave processing on the stability and antioxidant activity of delphinidin and petunidin. J. Food Biochem. 2019, 43, e12818. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization and role of polyphenol oxidase and peroxidase in browning of fresh-cut melon. J. Agric. Food Chem. 2007, 56, 132–138. [Google Scholar] [CrossRef]
- Bhagat, B.; Chakraborty, S. Potential of pulsed light treatment to pasteurize pomegranate juice: Microbial safety, enzymatic inactivation, and phytochemical retention. LWT—J. Food Sci. Technol. 2022, 159, 113215. [Google Scholar] [CrossRef]
- Etzbach, L.; Pfeiffer, A.; Schieber, A.; Weber, F. Effects of thermal pasteurization and ultrasound treatment on the peroxidase activity, carotenoid composition, and physicochemical properties of goldenberry (Physalis peruviana L.) puree. LWT—Food Sci. Technol. 2019, 100, 69–74. [Google Scholar] [CrossRef]
- Ijod, G.; Musa, F.N.; Anwar, F.; Suleiman, N.; Adzahan, N.M.; Azman, E.M. Thermal and nonthermal pretreatment methods for the extraction of anthocyanins: A review. J. Food Process. Preserv. 2022, 46, e17255. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Degradation kinetics of anthocyanins from purple rice bran and effect of hydrocolloids on its stability. J. Food Process. Eng. 2020, 43, e13360. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Zhang, H.; Mai, Q.; Zhang, B.; Li, H.; Deng, Z. The degradation rules of anthocyanins from eggplant peel and antioxidant capacity in fortified model food system during the thermal treatments. Food Biosci. 2020, 38, 100701. [Google Scholar] [CrossRef]
- Pan, F.; Li, J.; Zhao, L.; Tuersuntuoheti, T.; Mehmood, A.; Zhou, N.; Hao, S.; Wang, C.; Guo, Y.; Lin, W. A molecular docking and molecular dynamics simulation study on the interaction betweencyanidin-3-O-glucoside and major proteins in cow’s milk. J. Food Biochem. 2021, 45, e13570. [Google Scholar] [CrossRef] [PubMed]
- Rencher, A.C. Methods of Multivariate Analysis; John Wiley & Sons: New York, NY, USA, 2003; Volume 492. [Google Scholar]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V.; Swanson, B.G. High hydrostatic pressure processing of fruit and vegetable products. Food Rev. Int. 2005, 21, 411–425. [Google Scholar] [CrossRef]
- Yang, W.; Kaimainen, M.; Jarvenp, E.; Sandell, M.; Huopalahti, R.; Yang, B.; Laaksonen, O. Red beet (Beta vulgaris) betalains and grape (Vitis vinifera) anthocyanins as colorants in white currant juice—Effect of storage on degradation kinetics, color stability and sensory properties. Food Chem. 2021, 348, 128995. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.P.R.; Chisté, R.C.; Pena, R.S. Stability of ascorbic acid and anthocyanins of jambolan (Syzygium cumini) and camu-camu (Myrciaria dubia) juice blend during pasteurization and storage at room temperature. J. Food Process. Preserv. 2022, 46, e16509. [Google Scholar] [CrossRef]
- De Carvalho, I.T. Microbiologia dos alimentos. In Escola Técnica Aberta do Brasil, 1st ed.; EDUFRPE: Recife, Brazil, 2010; Chapter 8. [Google Scholar]
- Padrões de Identidade e Qualidade de suco e Polpa de Fruta—Instrução Normativa n◦ 49/2018; Regulamento Técnico Geral para fixação dos Padrões de Identidade e Qualidade para Polpa de Fruta e Suco de Fruta; Diário Oficial da União: Brasília, Brazil, 2018. Available online: https://www.in.gov.br/web/guest/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/42586576/do1-2018-09-27-instrucao-normativa-n-49-de-26- (accessed on 5 August 2023).
- Lacerda de Oliveira, L.; Sanchez, B.A.O.; Celestino, I.C.; Celestino, S.M.C.; Alencar, E.R.; Costa, A.M. Shelf life and retention of bioactive compounds in storage of pasteurized Passiflora setacea pulp, an exotic fruit from Brazilian savannah. LWT—Food Sci. Technol. 2022, 159, 113202. [Google Scholar] [CrossRef]
- Ağçam, E.; Akyıldız, A.; Dündar, B. Thermal pasteurization and microbial inactivation of fruit juices. In Fruit Juices—Extraction, Composition, Quality and Analysis; Rajauria, G., Tiwari, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 309–339. [Google Scholar] [CrossRef]




| Property/Component | Treatment | |
|---|---|---|
| Juice without CA | Juice with 1% CA | |
| Physicochemical properties | ||
| Total soluble solids, TSS (°Brix) | 7.0 ± 0.01 b | 8.0 ± 0.58 a |
| Total titratable acidity, TTA (g CA/100 mL) | 0.13 ± 0.01 b | 0.91 ± 0.02 a |
| pH | 4.4 ± 0.06 a | 3.2 ± 0.14 b |
| TSS/TTA | 55.87 ± 0.14 a | 8.74 ± 0.06 b |
| Total sugars (g/100 mL) | 9.48 ± 0.26 a | 9.45 ± 0.25 a |
| Bioactive compounds | ||
| Total anthocyanins (mg cyanidin 3-glucoside/L) | 13.69 ± 0.08 b | 18.77 ± 1.62 a |
| Total phenolic compounds (mg GAE/L) | 31.09 ± 1.45 b | 39.14 ± 1.51 a |
| Enzymatic activity | ||
| Peroxidase (U/mL) | 6.0 ± 0.70 a | 0.39 ± 0.04 b |
| Polyphenoloxidase (U/mL) | 8.0 ± 0.70 a | 5.21 ± 0.56 b |
| Property | Unpasteurized (Control) | Pasteurization Temperature | ||||
|---|---|---|---|---|---|---|
| 60 °C | 70 °C | 80 °C | 90 °C | 100 °C | ||
| Total soluble solids, TSS (°Brix) | 8.0 ± 0.58 a | 8.10 ± <0.01 a | 8.7 ± 0.12 a | 8.5 ± <0.01 a | 8.4 ± <0.01 a | 8.4 ± <0.01 a |
| Total titratable acidity, TTA (g CA/100 mL) | 0.91 ± 0.02 c | 1.77 ± 0.01 b | 2.09 ± 0.02 a | 1.65 ± 0.07 b | 1.65 ± <0.01 b | 1.83 ± 0.07 b |
| pH | 3.2 ± 0.14 a | 3.3 ± <0.01 a | 3.2 ± 0.06 a | 3.3 ± 0.06 a | 3.3 ± <0.01 a | 3.3 ± 0.06 a |
| TSS/TTA | 8.74 ± 0.06 a | 4.57 ± 0.21 c | 4.16 ± 0.08 d | 5.13 ± 0.21 b | 5.10 ± 0.01 b | 4.71 ± 0.18 c |
| Total anthocyanins (mg cyanidin 3-glucoside/L) | 18.77 ± 1.62 a | 13.20 ± 0.86 b | 12.47 ± 1.05 b | 10.93 ± 0.75 bc | 11.72 ± 0.13 bc | 9.05 ± 0.02 c |
| Total phenolic compounds (mg GAE/L) | 39.14 ± 1.51 a | 36.84 ± 4.21 a | 41.41 ± 0.54 a | 41.09 ± 1.30 a | 39.73 ± 0.64 a | 36.96 ± 0.47 a |
| Peroxidase, POD (U/mL) | 0.39 ± 0.04 b | 0.30 ± <0.01 b | 1.0 ± <0.01 a | nd | nd | nd |
| Polyphenol oxidase, PPO (U/mL) | 5.21 ± 0.56 a | 2.0 ± <0.01 b | 2.0 ± <0.01 b | nd | nd | nd |
| Anthocyanins | Unpasteurized (Control) | Pasteurization Temperature | ||||
|---|---|---|---|---|---|---|
| 60 °C | 70 °C | 80 °C | 90 °C | 100 °C | ||
| Peak 1—delphinidin 3-glucoside (mg/L) | 10.68 ± 0.60 a | 11.72 ± 0.29 a | 7.40 ± 0.07 b | 11.22 ± 0.08 a | 8.65 ± 0.32 b | 6.77 ± 0.72 c |
| Peak 2—Unidentified (mg cy-3-glu/L) | 4.51 ± 0.06 b | 5.43 ± 0.06 a | 3.35 ± 0.08 c | 4.94 ± 0.39 a | 4.08 ± 0.47 b | 3.42 ± 0.14 c |
| Peak 3—cyanidin 3-glucoside (mg/L) | 13.67 ± 0.04 a | 9.81 ± 0.08 c | 14.37 ± 0.09 a | 10.80 ± 0.18 b | 8.77 ± 0.28 c | 11.96 ± 0.84 b |
| Peak 4—Unidentified (mg cy-3-glu/L) | 17.53 ± 0.25 b | 12.77 ± 0.24 c | 24.76 ± 0.05 a | 14.99 ± 0.66 c | 17.85 ± 1.89 b | 27.68 ± 0.12 a |
| Total anthocyanins (mg cy-3-glu/L) | 46.39 ± 1.01 b | 39.73 ± 0.62 c | 49.88 ± 0.52 a | 41.95 ± 1.54 c | 39.35 ± 2.85 c | 49.83 ± 2.76 ab |
| Properties | Time (s) | ||
|---|---|---|---|
| 60 | 90 | 120 | |
| Total anthocyanins (mg cy 3-glu/L) | 11.27 ± 0.75 a | 9.34 ± 0.29 b | 11.56 ± 0.82 a |
| Total phenolic compounds (mg GAE/L) | 41.09 ± 1.30 a | 36.68 ± 0.83 a | 30.97 ± 1.47 b |
| Peroxidase (U/mL) | nd | nd | nd |
| Polyphenol oxidase (U/mL) | nd | nd | nd |
| Microorganism Group | Grumixama Juice | |
|---|---|---|
| Unpasteurized | Pasteurized | |
| Coliforms at 35 °C (CFU/mL) | <3.0 | <3.0 |
| Coliforms at 45 °C (CFU/mL) | <3.0 | <3.0 |
| Aerobic mesophiles (CFU/mL) | 1.84 × 103 ± <0.01 | <1.0 |
| Fungi (CFU/mL) | <1.0 | <1.0 |
| Staphylococcus aureus (log CFU/L) | 1.80 × 102 ± <0.01 | <1.0 |
| Salmonella sp. | Absence | Absence |
| Properties | Room Temperature (25 ± 1 °C) | Chilling Temperature (7 ± 1 °C) | ||||
|---|---|---|---|---|---|---|
| Time (Days) | Time (Days) | |||||
| 0 | 15 | 30 | 0 | 15 | 30 | |
| Physicochemical and chemical properties | ||||||
| Total soluble solids, TSS (°Brix) | 9.60 ± <0.01 a | 5.1 ± 0.06 b | 4.8 ± <0.01 c | 8.9 ± 0.06 a | 8.2 ± <0.01 c | 8.6 ± 0.06 b |
| Total titratable acidity, TTA (g CA/100 mL) | 2.42 ± 0.07 a | 1.38 ± 0.17 b | 1.17 ± 0.12 b | 0.89 ± 0.02 b | 1.19 ± 0.09 a | 0.96 ± 0.06 ab |
| pH | 3.3 ± 0.06 b | 4.0 ± 0.06 ab | 3.9 ± 0.29 a | 3.7 ± 0.06 a | 3.4 ± <0.01 b | 3.2 ± 0.02 c |
| Retention of total anthocyanins (%) | 100 ± 0.62 a | 60.31 ± 2.69 b | 57.51 ± 2.13 b | 100 ± 1.88 a | 114 ± 1.37 a | 62.14 ± 0.02 b |
| Retention of total phenolic compounds (%) | 100 ± 0.97 a | 84.69 ± 3.46 b | 69.47 ± 1.38 c | 100 ± 0.90 a | 91.28 ± 1.44 a | 71.49 ± 2.19 b |
| Enzymatic activity | ||||||
| Peroxidase (U/mL) | nd | nd | nd | nd | nd | nd |
| Polyphenol oxidase (U/mL) | nd | nd | nd | nd | nd | nd |
| Microbiological standard | ||||||
| Aerobic mesophiles (CFU/mL) | <1.0 | <1.0 | 2.6 × 102 ± <0.01 | <1.0 | <1.0 | <1.0 |
| Fungi (CFU/mL) | <1.0 | 1.86 × 104 ± 1.12 | 1.30 × 105 ± 1.02 | <1.0 | <1.0 | <1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modesto Junior, E.N.; Chaves, R.P.F.; Martins, M.G.; Pereira, G.A.; Chisté, R.C.; Pena, R.d.S. Phenolic Compounds Stability of Grumixama (Eugenia brasiliensis) Juice during Processing and Storage. Beverages 2023, 9, 91. https://doi.org/10.3390/beverages9040091
Modesto Junior EN, Chaves RPF, Martins MG, Pereira GA, Chisté RC, Pena RdS. Phenolic Compounds Stability of Grumixama (Eugenia brasiliensis) Juice during Processing and Storage. Beverages. 2023; 9(4):91. https://doi.org/10.3390/beverages9040091
Chicago/Turabian StyleModesto Junior, Elivaldo Nunes, Rosane Patricia Ferreira Chaves, Mayara Galvão Martins, Gustavo Araujo Pereira, Renan Campos Chisté, and Rosinelson da Silva Pena. 2023. "Phenolic Compounds Stability of Grumixama (Eugenia brasiliensis) Juice during Processing and Storage" Beverages 9, no. 4: 91. https://doi.org/10.3390/beverages9040091
APA StyleModesto Junior, E. N., Chaves, R. P. F., Martins, M. G., Pereira, G. A., Chisté, R. C., & Pena, R. d. S. (2023). Phenolic Compounds Stability of Grumixama (Eugenia brasiliensis) Juice during Processing and Storage. Beverages, 9(4), 91. https://doi.org/10.3390/beverages9040091