Validation of Perioperative Troponin Levels for Predicting Postoperative Mortality and Long-Term Survival in Patients Undergoing Surgery for Hepatobiliary and Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Endpoints
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Validation of Perioperative TnT Levels
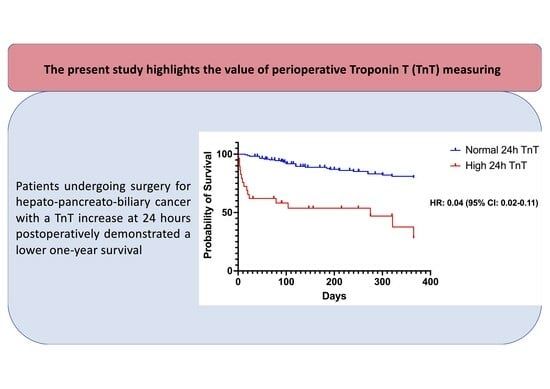
3.3. Evaluation of TnT Levels in Terms of Long-Term Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Moonesinghe, S.R.; Mythen, M.G.; Das, P.; Rowan, K.M.; Grocott, M.P.W. Risk stratification tools for predicting morbidity and mortality in adult patients undergoing major surgery: Qualitative systematic review. Anesthesiology 2013, 119, 959–981. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Liu, Y.; Paruch, J.L.; Zhou, L.; Kmiecik, T.E.; Ko, C.Y.; Cohen, M.E. Development and Evaluation of the Universal ACS NSQIP Surgical Risk Calculator: A Decision Aid and Informed Consent Tool for Patients and Surgeons. J. Am. Coll. Surg. 2013, 217, 833–842.e3. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gupta, H.; Sundaram, A.; Kaushik, M.; Fang, X.; Miller, W.J.; Esterbrooks, D.J.; Hunter, C.B.; Pipinos, I.I.; Johanning, J.M.; et al. Development and Validation of a Risk Calculator for Prediction of Cardiac Risk after Surgery. Circulation 2011, 124, 381–387. [Google Scholar] [CrossRef]
- Protopapa, K.L.; Simpson, J.C.; E Smith, N.C.; Moonesinghe, S.R. Development and validation of the Surgical Outcome Risk Tool (SORT). Br. J. Surg. 2014, 101, 1774–1783. [Google Scholar] [CrossRef]
- Karamolegkou, A.P.; Fergadi, M.P.; Magouliotis, D.E.; Samara, A.A.; Tatsios, E.; Xanthopoulos, A.; Pourzitaki, C.; Walker, D.; Zacharoulis, D. Validation of the Surgical Outcome Risk Tool (SORT) and SORT v2 for Predicting Postoperative Mortality in Patients with Pancreatic Cancer Undergoing Surgery. J. Clin. Med. 2023, 12, 2327. [Google Scholar] [CrossRef]
- Botto, F.; Alonso-Coello, P.; Chan, M.T.; Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group. Myocardial injury after noncardiac surgery: A large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014, 120, 564–578. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577, Erratum in Ann. Intern. Med. 2008, 148, 168. PMID: 17938396. [Google Scholar] [CrossRef]
- Wong, D.J.N.; Harris, S.; Sahni, A.; Bedford, J.R.; Cortes, L.; Shawyer, R.; Wilson, A.M.; Lindsay, H.A.; Campbell, D.; Popham, S.; et al. Developing and validating subjective and objective risk-assessment measures for predicting mortality after major surgery: An international prospective cohort study. PLoS Med. 2020, 17, e1003253. [Google Scholar] [CrossRef]
- Ruetzler, K.; Smilowitz, N.R.; Berger, J.S.; Devereaux, P.; Maron, B.A.; Newby, L.K.; Perez, V.d.J.; Sessler, D.I.; Wijeysundera, D.N. Diagnosis and Management of Patients with Myocardial Injury after Noncardiac Surgery: A Scientific Statement from the American Heart Association. Circulation 2021, 144, E287–E305. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating charac-teristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Humble, C.A.S.; Huang, S.; Jammer, I.; Björk, J.; Chew, M.S. Prognostic performance of preoperative cardiac troponin and perioperative changes in cardiac troponin for the prediction of major adverse cardiac events and mortality in noncardiac surgery: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0215094. [Google Scholar] [CrossRef]
- Xue, F.S.; Cheng, Y.; Li, R.P. Use of preoperative cardiac troponin T to identify patients at risk for acute myocardial infarction and long-term mortality after major noncardiac surgery. Am. Heart J. 2014, 167, e5. [Google Scholar] [CrossRef]
- Zahid, J.A.; Orhan, A.; Hadi, N.A.-H.; Ekeloef, S.; Gögenur, I. Myocardial injury and long-term oncological outcomes in patients undergoing surgery for colorectal cancer. Int. J. Color. Dis. 2023, 38, 234. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Szczeklik, W. Myocardial injury after non-cardiac surgery: Diagnosis and management. Eur. Heart J. 2019, 41, 3083–3091. [Google Scholar] [CrossRef]
- Cohen, M.C.; Aretz, T.H. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc. Pathol. 1999, 8, 133–139. [Google Scholar] [CrossRef]
- Ellis, S.G.; Hertzer, N.R.; Young, J.R.; Brener, S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am. J. Cardiol. 1996, 77, 1126–1128. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, e278–e333. [Google Scholar]
- Kristensen, S.D.; Knuuti, J. New ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management. Eur. Heart J. 2014, 35, 2344–2345. [Google Scholar] [CrossRef]
- Duceppe, E.; Parlow, J.; MacDonald, P.; Lyons, K.; McMullen, M.; Srinathan, S.; Graham, M.; Tandon, V.; Styles, K.; Bessissow, A.; et al. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can. J. Cardiol. 2016, 33, 17–32. [Google Scholar] [CrossRef]
- Writing Committee for the VISION Study Investigators; Devereaux, P.J.; Biccard, B.M.; Sigamani, A.; Xavier, D.; Chan, M.T.V.; Srinathan, S.K.; Walsh, M.; Abraham, V.; Pearse, R.; et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017, 317, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Torborg, A.; Ryan, L.; Kantor, G.; Biccard, B.M. The pharmacoeconomics of routine postoperative troponin surveillance to prevent and treat myocardial infarction after non-cardiac surgery. S. Afr. Med. J. 2014, 104, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]



| Demographics | Number of Patients, n = 195 |
|---|---|
| Female, n (%) | 49 (25.1) |
| Mean age, years (SD) | 64.2 (11.4) |
| Age ≥ 70 (%) | 60 (30.8) |
| BMI, (SD) | 26.5 (1.9) |
| Mean previous operations, n (SD) | 1.9 (1) |
| ASA class, n (%) | |
| I | 38 (19.4) |
| II | 98 (50.3) |
| III | 44 (22.6) |
| IV | 15 (7.7) |
| SORT (SD) | 1.35 (1.9) |
| Operative priority | |
| Elective | 158 (81.0) |
| Acute | 37 (19.0) |
| Cancer site, n (%) | |
| Pancreas | 164 (84.1) |
| Stage | |
| Resectable | 110 (67.1) |
| Borderline resectable | 54 (32.9) |
| PDAC | 139 (84.8) |
| NET | 14 (8.5) |
| Other | 11 (6.7) |
| Hepatobiliary | 31 (15.9) |
| Stage | |
| I, II | 25 (80.6) |
| III | 6 (19.4) |
| Neoadjuvant treatment, n (%) | 87 (44.6) |
| Surgical operation, n (%) | |
| Pancreaticoduodenectomy | 83 (42.6) |
| Distal pancreatectomy | 12 (6.2) |
| Hepatectomy | 32 (16.4) |
| Other procedures | 68 (34.8) |
| Blood loss, n (%) | |
| <100 mL | 61 (31.3) |
| 101–500 mL | 92 (47.2) |
| 501–1000 mL | 36 (18.5) |
| >1001 mL | 6 (3) |
| Anesthesia events, n (%) | 9 (4.6) |
| Severity of procedure, n (%) | |
| Major/Complex | 182 (93.3) |
| 30-day mortality | 13 (6.6) |
| Predictive Marker | O | E | O:E | Discrimination | Calibration | ||
|---|---|---|---|---|---|---|---|
| AUC (95% CI) | p | H-L | p | ||||
| Preoperative TnT | 13 | 0 | - | 0.60 (0.42–0.77) | 0.242 | 1 | 0.32 |
| TnT at 24 h postoperatively | 13 | 5 | 2.6 | 0.88 (0.82–0.93) | <0.001 | 1.38 | 0.24 |
| TnT at 48 h postoperatively | 12 | 2 | 6 | 0.75 (0.57–0.92) | 0.004 | 2.7 | 0.1 |
| Odds Ratio | 95% Confidence Intervals | |
|---|---|---|
| Unadjusted TnT at 24 h postoperatively | 0.88 | 0.82–0.93 |
| Adjusted * TnT at 24 h postoperatively | 0.87 | 0.77–0.97 |
| Demographics | Pre-op TnT | TnT 24 h | TnT 48 h | |||
|---|---|---|---|---|---|---|
| Normal n = 189 | High n = 6 | Normal n = 166 | High n = 29 | Normal n = 167 | High n = 27 | |
| Female, n (%) | 48 (25.4) | 1 (16.7) | 42 (25.3) | 7 (24.1) | 42 (25.2) | 7 (25.9) |
| Age ≥ 70 (%) | 58 (30.7) | 2 (33.3) | 45 (27.1) | 15 (51.7) | 45 (26.9) | 14 (51.9) |
| ASA Class, n (%) | ||||||
| I | 38 (20.1) | 0 (0) | 36 (21.7) | 2 (6.9) | 36 (21.6) | 2 (7.4) |
| II | 97 (51.3) | 1 (16.7) | 90 (54.2) | 8 (27.6) | 90 (53.9) | 8 (29.6) |
| III | 41 (21.7) | 3 (50) | 32 (19.3) | 12 (41.4) | 32 (19.2) | 12 (44.4) |
| IV | 13 (6.9) | 2 (33.3) | 8 (4.8) | 7 (24.1) | 8 (4.8) | 6 (22.2) |
| SORT (SD) | 1.3 (1.7) | 4.5 (4.3) | 1.1 (1.3) | 2.7 (3.6) | 1.2 (1.5) | 2.7 (3.4) |
| Operative priority, n (%) | ||||||
| Elective | 156 (82.5) | 2 (33.3) | 142 (85.5) | 16 (55.2) | 142 (85.0) | 16 (59.3) |
| Acute | 33 (17.5) | 4 (66.6) | 24 (14.5) | 13 (44.8) | 24 (14.4) | 12 (44.4) |
| Cancer site, n (%) | ||||||
| Pancreas | 161 (85.2) | 3 (50) | 144 (86.7) | 20 (69) | 144 (86.2) | 19 (70.4) |
| Stage | ||||||
| Resectable | 118 (73.3) | 2 (33.3) | 103 (62.0) | 7 (24.1) | 103 (61.7) | 7 (25.9) |
| Borderline resectable | 53 (32.9) | 1 (16.7) | 41 (24.7) | 13 (44.8) | 41 (24.6) | 12 (44.4) |
| PDAC | 136 (84.5) | 3 (50) | 121 (72.9) | 18 (62) | 121 (72.5) | 17 (63.0) |
| NET | 14 (8.7) | 0 (0) | 12 (7.2) | 2 (6.9) | 12 (7.2) | 2 (7.4) |
| Other | 11 (6.8) | 0 (0) | 11 (6.6) | 0 (0) | 11 (6.6) | 0 (0) |
| Hepatobiliary | 28 (14.8) | 3 (50) | 22 | 9 | 22 | 9 |
| Stage | ||||||
| I, II | 23 (82.1) | 2 (66.7) | 21 (12.7) | 4 (13.8) | 21 (12.6) | 4 (14.8) |
| III | 5 (17.9) | 1 (33.3) | 1 (0.6) | 5 (17.2) | 1 (0.6) | 5 (18.5) |
| Neoadjuvant treatment, n (%) | 85 (45.0) | 2 (66.7) | 73 (44.0) | 14 (48.3) | 73 (43.7) | 14 (51.9) |
| Surgical operation, n (%) | ||||||
| Pancreaticoduodenectomy | 80 (42.4) | 3 (50) | 66 (39.8) | 17 (58.6) | 66 (39.5) | 16 (59.3) |
| Distal pancreatectomy | 12 (6.3) | 0 (0) | 10 (6.0) | 2 (6.9) | 10 (6.0) | 2 (7.4) |
| Hepatectomy | 29 (15.3) | 3 (50) | 23 (13.9) | 9 (31.0) | 23 (13.8) | 9 (33.3) |
| Other procedures | 68 (36.0) | 0 (0) | 67 (40.4) | 1 (3.4) | 67 (40.1) | 1 (3.7) |
| Blood loss | ||||||
| <100 mL | 61 (32.3) | 0 (0) | 60 (36.1) | 1 (3.4) | 60 (35.9) | 1 (3.7) |
| 101–500 mL | 89 (47.1) | 3 (50) | 78 (47.0) | 14 (48.3) | 78 (46.7) | 14 (51.9) |
| 501–1000 mL | 35 (18.5) | 1 (16.7) | 25 (15.1) | 11 (37.9) | 25 (15.0) | 11 (40.7) |
| >1001 mL | 7 (3.7) | 2 (33.3) | 6 (3.6) | 3 (10.3) | 6 (3.6) | 2 (7.4) |
| Anesthesia events, n (%) | 6 (3.2) | 3 (50) | 5 (3.0) | 4 (13.8) | 5 (3.0) | 3 (11.1) |
| Severity of procedure, n (%) | ||||||
| Major/Complex | 178 (94.2) | 4 (66.6) | 158 (95.2) | 24 (82.8) | 158 (94.6) | 23 (85.2) |
| 30-day mortality | 11 (5.8) | 2 (33.3) | 3 (1.8) | 10 (34.5) | 3 (1.8) | 9 (33.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magouliotis, D.E.; Tatsios, E.; Giamouzis, G.; Samara, A.A.; Xanthopoulos, A.; Briasoulis, A.; Skoularigis, J.; Athanasiou, T.; Bareka, M.; Kourek, C.; et al. Validation of Perioperative Troponin Levels for Predicting Postoperative Mortality and Long-Term Survival in Patients Undergoing Surgery for Hepatobiliary and Pancreatic Cancer. J. Cardiovasc. Dev. Dis. 2024, 11, 130. https://doi.org/10.3390/jcdd11040130
Magouliotis DE, Tatsios E, Giamouzis G, Samara AA, Xanthopoulos A, Briasoulis A, Skoularigis J, Athanasiou T, Bareka M, Kourek C, et al. Validation of Perioperative Troponin Levels for Predicting Postoperative Mortality and Long-Term Survival in Patients Undergoing Surgery for Hepatobiliary and Pancreatic Cancer. Journal of Cardiovascular Development and Disease. 2024; 11(4):130. https://doi.org/10.3390/jcdd11040130
Chicago/Turabian StyleMagouliotis, Dimitrios E., Evangelos Tatsios, Grigorios Giamouzis, Athina A. Samara, Andrew Xanthopoulos, Alexandros Briasoulis, John Skoularigis, Thanos Athanasiou, Metaxia Bareka, Christos Kourek, and et al. 2024. "Validation of Perioperative Troponin Levels for Predicting Postoperative Mortality and Long-Term Survival in Patients Undergoing Surgery for Hepatobiliary and Pancreatic Cancer" Journal of Cardiovascular Development and Disease 11, no. 4: 130. https://doi.org/10.3390/jcdd11040130
APA StyleMagouliotis, D. E., Tatsios, E., Giamouzis, G., Samara, A. A., Xanthopoulos, A., Briasoulis, A., Skoularigis, J., Athanasiou, T., Bareka, M., Kourek, C., & Zacharoulis, D. (2024). Validation of Perioperative Troponin Levels for Predicting Postoperative Mortality and Long-Term Survival in Patients Undergoing Surgery for Hepatobiliary and Pancreatic Cancer. Journal of Cardiovascular Development and Disease, 11(4), 130. https://doi.org/10.3390/jcdd11040130