Does Anatomic Phenotype of Mitral Annular Disjunction Impact Survival? An Autopsy-Based Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Gross Examination
2.3. Statistical Analysis
3. Results
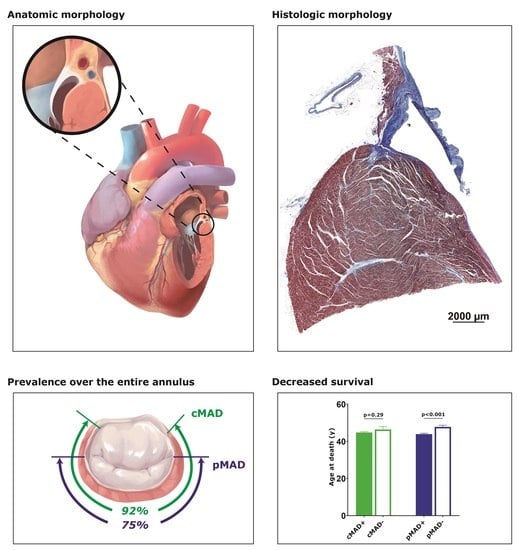
3.1. Longitudinal and Circumferential Distributions of MAD
3.2. pMAD+ Was Associated with Younger Age at Death
3.3. Circumferential and Longitudinal Extent Was Associated with Morphological Changes
4. Discussion
4.1. MAD Is Common along the Entire Mitral Annulus
4.2. Pathogenicity of pMAD
4.3. Length and Distribution of pMAD Are Predictors for Pathogenicity
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hutchins, G.M.; Moore, G.W.; Skoog, D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N. Engl. J. Med. 1986, 314, 535–540. [Google Scholar] [CrossRef]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef] [Green Version]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Dejgaard, L.A.; Skjolsvik, E.T.; Lie, O.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The mitral annulus disjunction arrhythmic syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Konda, T.; Tani, T.; Suganuma, N.; Nakamura, H.; Sumida, T.; Fujii, Y.; Kawai, J.; Kitai, T.; Kim, K.; Kaji, S.; et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J. Echocardiogr. 2017, 15, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, V.; Tamborini, G.; Muratori, M.; Gripari, P.; Fusini, L.; Italiano, G.; Volpato, V.; Sassi, V.; Pepi, M. Mitral annular disjunction in a large cohort of patients with mitral valve prolapse and significant regurgitation. JACC Cardiovasc. Imaging 2019, 12, 2278–2280. [Google Scholar] [CrossRef]
- Essayagh, B.; Iacuzio, L.; Civaia, F.; Avierinos, J.F.; Tribouilloy, C.; Levy, F. Usefulness of 3-tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am. J. Cardiol. 2019, 124, 1725–1730. [Google Scholar] [CrossRef]
- Eriksson, M.J.; Bitkover, C.Y.; Omran, A.S.; David, T.E.; Ivanov, J.; Ali, M.J.; Woo, A.; Siu, S.C.; Rakowski, H. J. Am. Soc. Echocardiogr. 2005, 18, 1014–1022. [CrossRef]
- Lee, A.P.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional implication of mitral annular disjunction in mitral valve prolapse: A quantitative dynamic 3D echocardiographic study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Konda, T.; Tani, T.; Suganuma, N.; Fujii, Y.; Ota, M.; Kitai, T.; Kaji, S.; Furukawa, Y. Mitral annular disjunction in patients with primary severe mitral regurgitation and mitral valve prolapse. Echocardiography 2020, 37, 1716–1722. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ghulam Ali, S.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2020, 107, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Putnam, A.J.; Kebed, K.; Mor-Avi, V.; Rashedi, N.; Sun, D.; Patel, B.; Balkhy, H.; Lang, R.M.; Patel, A.R. Prevalence of mitral annular disjunction in patients with mitral valve prolap.pse and severe regurgitation. Int. J. Cardiovasc. Imaging 2020, 36, 1363–1370. [Google Scholar] [CrossRef]
- Tsianaka, T.; Matziris, I.; Kobe, A.; Euler, A.; Kuzo, N.; Erhart, L.; Leschka, S.; Manka, R.; Kasel, A.M.; Tanner, F.C.; et al. Mitral annular disjunction in patients with severe aortic stenosis: Extent and reproducibility of measurements with computed tomography. Eur. J. Radiol. Open 2021, 8, 100335. [Google Scholar] [CrossRef]
- Toh, H.; Mori, S.; Izawa, Y.; Fujita, H.; Miwa, K.; Suzuki, M.; Takahashi, Y.; Toba, T.; Watanabe, Y.; Kono, A.K.; et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: Comprehensive 3D analysis using cardiac computed tomography. Eur. Heart J.-Cardiovasc. Imaging 2021, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Ho, S.Y.; Anderson, R.H.; Davies, M.J.; Becker, A.E. A histological study of the atrioventricular junction in hearts with normal and prolapsed leaflets of the mitral valve. Br. Heart J. 1988, 59, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.Y. Anatomy of the mitral valve. Heart 2002, 88 (Suppl. 4), iv5–iv10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, A. Cardiac valve surgery—The “French correction”. J. Thorac. Cardiovasc. Surg. 1983, 86, 323–337. [Google Scholar] [CrossRef]
- Punjabi, P.P.; Rana, B.S. Mitral annular disjunction: Is MAD ‘normal’. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 623–625. [Google Scholar] [CrossRef]
- Sugiura, M.; Ohkawa, S.; Watanabe, C.; Toku, A.; Imai, T.; Kuboki, K.; Shimada, H. Morphological observation of the mitral annulus fibrosus in patients with mitral valve prolapse. J. Cardiol. Suppl. 1990, 23, 21–28; discussion 29–30. [Google Scholar]
- Nayak, V.M.; Victor, S. Sub-mitral membranous curtain: A potential anatomical basis for congenital sub-mitral aneurysms. Indian J. Thorac. Cardiovasc. Surg. 2006, 22, 205–211. [Google Scholar] [CrossRef]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Caretta, A.; Viani, G.M.; Schlossbauer, S.A.; Demertzis, S.; Ho, S.Y. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Zhao, Q.; Zeng, X.; Zheng, D.; Yue, J.; Zhang, K.; Mao, D.; Makielski, J.C.; Cheng, J. Association of Mitral Annular Disjunction With Premature Cardiac Mortality in a Lar.r.rge Series of Autopsies. J. Am. Coll. Cardiol. 2021, 77, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Aabel, E.W.; Chivulescu, M.; Dejgaard, L.A.; Ribe, M.; Gjertsen, E.; Hopp, E.; Hunt, T.E.; Lie, O.H.; Haugaa, K.H. Tricuspid Annulus Disjunction: Novel Findings by Cardiac Magnetic Resonance in Patients With Mitral Annulus Disjunction. JACC Cardiovasc. Imaging 2021, 14, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Chivulescu, M.; Krohg-Sorensen, K.; Scheirlynck, E.; Lindberg, B.R.; Dejgaard, L.A.; Lie, O.H.; Helle-Valle, T.; Skjolsvik, E.T.; Estensen, M.E.; Edvardsen, T.; et al. Mitral annulus disjunction is associated with adverse outcome in Marfan and Loeys-Dietz syndromes. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 1035–1044. [Google Scholar] [CrossRef]
- Demolder, A.; Timmermans, F.; Duytschaever, M.; Muino-Mosquera, L.; De Backer, J. Association of Mitral Annular Disjunction with Cardiovascular Outcomes among Patients with Marfan Syndrome. JAMA Cardiol. 2021, 6, 1177–1186. [Google Scholar] [CrossRef]


| Cut-Off Value | AC a | P1 | P2 | P3 | PC a | cMAD+ | pMAD+ |
|---|---|---|---|---|---|---|---|
| ≥1.5 mm | 824/1251 (65.9) | 678/1373 (49.4) | 623/1373 (45.4) | 608/1373 (44.3) | 913/1228 (74.3) | 1264/1373 (92.1) | 1028/1373 (74.9) |
| ≥2 mm | 640/1251 (51.1) | 513/1373 (37.3) | 536/1373 (39.1) | 493/1373 (35.9) | 724/1228 (58.9) | 1151/1373 (83.8) | 897/1373 (65.3) |
| ≥2.5 mm | 362/1251 (28.9) | 301/1373 (21.9) | 359/1373 (26.1) | 276/1373 (20.1) | 441/1228 (35.9) | 843/1373 (61.3) | 608/1373 (44.2) |
| ≥3 mm | 176/1251 (14.0) | 160/1373 (11.6) | 207/1373 (15.1) | 155/1373 (11.3) | 256/1228 (20.1) | 558/1373 (40.6) | 377/1373 (27.4) |
| ≥4 mm | 43/1251 (3.4) | 25/1373 (1.8) | 60/1373 (4.4) | 43/1373 (3.1) | 78/1228 (6.3) | 181/1373 (13.2) | 108/1373 (7.9) |
| Total (n = 1373) | pMAD+ (n = 1028) | pMAD− (n = 345) | p | |
|---|---|---|---|---|
| Age at death (y) | 44.9 ± 0.4 | 43.9 ± 0.5 | 47.8 ± 0.9 | <0.001 |
| Men (%) | 1017 (74.1) | 771 (75.1) | 246 (71.3) | 0.18 |
| Height (cm) | 164.5 ± 0.2 | 164.8 ± 0.3 | 163.4 ± 0.4 | 0.006 |
| Anatomy of heart | ||||
| Weight of heart (g) | 380.6 ± 2.7 | 379.0 ± 3.1 | 385.4 ± 5.4 | 0.31 |
| Thickness of left ventricular wall (cm) | 1.23 ± 0.01 | 1.22 ± 0.01 | 1.24 ± 0.01 | 0.10 |
| Thickness of right ventricular wall (cm) | 0.31 ± 0.002 | 0.31 ± 0.003 | 0.32 ± 0.004 | 0.37 |
| Circumference of tricuspid annulus (cm) | 11.45 ± 0.02 | 11.45 ± 0.03 | 11.43 ± 0.04 | 0.62 |
| Circumference of pulmonary annulus (cm) | 7.82 ± 0.02 | 7.83 ± 0.03 | 7.80 ± 0.04 | 0.60 |
| Circumference of mitral annulus (cm) | 9.26 ± 0.03 | 9.25 ± 0.03 | 9.26 ± 0.05 | 0.79 |
| Circumference of aortic annulus (cm) | 7.02 ± 0.02 | 7.03 ± 0.02 | 7.00 ± 0.04 | 0.50 |
| Underlying cardiovascular conditions | ||||
| Coronary atherosclerosis (%) | 573 (41.7) | 411 (40.0) | 162 (47.0) | 0.03 b |
| Thoracic aortic aneurysm/dissection (%) | 66 (4.8) | 53 (5.2) | 13 (3.8) | 0.38 |
| Cardiomyopathies (%) | 74 (5.4) | 57 (5.5) | 17 (4.9) | 0.78 |
| Otherwise normal heart and vessel (%) | 517 (37.7) | 395 (38.4) | 122 (35.4) | 0.34 |
| Cause of death | ||||
| Diseases-dominant death (%) a | 1026 (74.7) | 764 (74.5) | 262 (75.9) | 0.57 |
| Cardiovascular | 565 (41.2) | 423 (41.2) | 142 (41.2) | >0.99 |
| Respiratory | 90 (6.6) | 81 (7.9) | 9 (2.6) | <0.001 |
| Digestive | 67 (4.9) | 42 (4.1) | 25 (7.3) | 0.03 |
| Violence-dominant death (%) a | 338 (24.6) | 257 (25.0) | 81 (23.5) | 0.61 |
| Trauma | 170 (12.4) | 122 (11.9) | 48 (13.9) | 0.34 |
| Poisoning | 79 (5.8) | 58 (5.6) | 21 (6.1) | 0.79 |
| Asphyxia | 59 (4.3) | 50 (4.9) | 9 (2.6) | 0.09 |
| pMAD− | Extensive Longitudinal Extent (Tri-Region pMAD) | Extensive Circumferential Extent (Mean Standardized Length > 1.78) | |
|---|---|---|---|
| n | 345 | 279 | 274 |
| Weight of heart (g) | 385.4 ± 5.4 | 387.8 ± 6.3 | 373.0 ± 5.4 |
| Thickness of left ventricular wall (cm) | 1.24 ± 0.01 | 1.24 ± 0.01 | 1.20 ± 0.01 a |
| Thickness of right ventricular wall (cm) | 0.32 ± 0.004 | 0.32 ± 0.007 | 0.30 ± 0.005 |
| Circumference of tricuspid annulus (cm) | 11.43 ± 0.04 | 11.64 ± 0.05 b | 11.49 ± 0.05 |
| Circumference of pulmonary annulus (cm) | 7.80 ± 0.04 | 7.98 ± 0.05 b | 7.90 ± 0.05 |
| Circumference of mitral annulus (cm) | 9.27 ± 0.05 | 9.46 ± 0.06 b | 9.35 ± 0.06 |
| Circumference of aortic annulus (cm) | 7.00 ± 0.04 | 7.19 ± 0.05 b | 7.17 ± 0.05 a |
| Coronary atherosclerosis (%) | 162 (47.0) | 118 (42.3) | 100 (36.5) |
| Thoracic aortic aneurysm/dissection (%) | 13 (3.8) | 26 (9.3) c | 25 (9.1) c |
| Cardiomyopathies (%) | 17 (4.9) | 17 (6.1) | 11 (4.0) |
| Otherwise normal heart and vessel (%) | 122 (35.4) | 96 (34.4) | 109 (39.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Zhao, Q.; Li, R.; Zheng, D.; Xiao, Y.; Mao, D.; Wang, Y.; Yue, J.; Zhang, K.; Makielski, J.C.; et al. Does Anatomic Phenotype of Mitral Annular Disjunction Impact Survival? An Autopsy-Based Retrospective Study. J. Cardiovasc. Dev. Dis. 2021, 8, 174. https://doi.org/10.3390/jcdd8120174
Zhou N, Zhao Q, Li R, Zheng D, Xiao Y, Mao D, Wang Y, Yue J, Zhang K, Makielski JC, et al. Does Anatomic Phenotype of Mitral Annular Disjunction Impact Survival? An Autopsy-Based Retrospective Study. Journal of Cardiovascular Development and Disease. 2021; 8(12):174. https://doi.org/10.3390/jcdd8120174
Chicago/Turabian StyleZhou, Nan, Qianhao Zhao, Rui Li, Da Zheng, Yuxi Xiao, Danmi Mao, Yunyi Wang, Jiacheng Yue, Kai Zhang, Jonathan C. Makielski, and et al. 2021. "Does Anatomic Phenotype of Mitral Annular Disjunction Impact Survival? An Autopsy-Based Retrospective Study" Journal of Cardiovascular Development and Disease 8, no. 12: 174. https://doi.org/10.3390/jcdd8120174
APA StyleZhou, N., Zhao, Q., Li, R., Zheng, D., Xiao, Y., Mao, D., Wang, Y., Yue, J., Zhang, K., Makielski, J. C., & Cheng, J. (2021). Does Anatomic Phenotype of Mitral Annular Disjunction Impact Survival? An Autopsy-Based Retrospective Study. Journal of Cardiovascular Development and Disease, 8(12), 174. https://doi.org/10.3390/jcdd8120174