Endolichenic Fungi: A Promising Medicinal Microbial Resource to Discover Bioactive Natural Molecules—An Update
Abstract
:1. Introduction
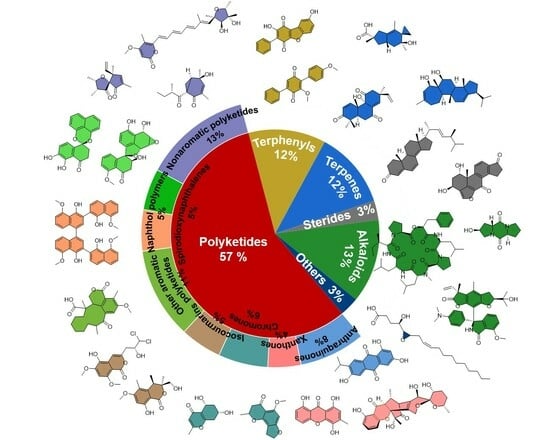
2. Different Types of Natural Products from Endolichenic Fungi
2.1. Polyketides
2.1.1. Simple Aromatic Polyketides
2.1.2. Simple Nonaromatic Polyketides
2.1.3. Complex Aromatic Polyketides
2.2. Polyphenyls
2.3. Terpenoids
2.4. Alkaloids and Their Biological Activities
2.5. Steroids
2.6. Others
3. A Meta-Analysis of Research Progress and Status of Endolichenic Fungi
3.1. The Main Research Groups Engaged in the Study of Endolichenic Fungi
3.2. The Fungal Sources and Structural Characteristics of the Isolated Secondary Metabolites
3.3. The Biological Activity of the Secondary Metabolites of Endolichenic Fungi
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef]
- Wei, J.C.; Abbas, A. The lichen genus Pseudevernia zope in China. Mycosystema 2003, 22, 26–29. [Google Scholar]
- Schweiger, A.H.; Ullmann, G.M.; Nürk, N.M.; Triebel, D.; Schobert, R.; Rambold, G. Chemical properties of key metabolites determine the global distribution of lichens. Ecol. Lett. 2022, 25, 416–426. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef] [PubMed]
- Boustie, J.; Tomasi, S.; Grube, M. Bioactive lichen metabolites: Alpine habitats as an untapped source. Phytochem. Rev. 2010, 10, 287–307. [Google Scholar] [CrossRef]
- de Vera, J.-P.; Horneck, G.; Rettberg, P.; Ott, S. The potential of the lichen symbiosis to cope with extreme conditions of outer space–I. Influence of UV radiation and space vacuum on the vitality of lichen symbiosis and germination capacity. Int. J. Astrobiol. 2003, 1, 285–293. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, E.H.; Lee, H.K.; Hong, S.G. Biodiversity and physiological characteristics of Antarctic and Arctic lichens-associated bacteria. World J. Microbiol. Biotechnol. 2014, 30, 2711–2721. [Google Scholar] [CrossRef]
- Seymour, F.A.; Crittenden, P.D.; Dickinson, M.J.; Paoletti, M.; Montiel, D.; Cho, L.; Dyer, P.S. Breeding systems in the lichen-forming fungal genus Cladonia. Fungal Genet. Biol. 2005, 42, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-protectant metabolites from lichens and their symbiotic partners. Nat. Prod. Rep. 2013, 30, 1490–1508. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Daba, G.M. Lichens, an alternative drug for modern diseases. Int. J. Res. Pharm. Biosci. 2019, 6, 5–9. [Google Scholar]
- Romagni, J.G.; Dayan, F.E. Structural diversity of lichen metabolites and their potential use. In Advances in Microbial Toxin Research and Its Biotechnological Exploitation; Upadhyay, R.K., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 151–169. [Google Scholar]
- Huneck, S.; Yoshinmura, I. Identification of lichen substances. In Identification of Lichen Substances; Springer: Berlin/Heidelberg, Germany, 1996; pp. 11–123. [Google Scholar]
- Rankovic, B.; Kosanic, M. Lichens as a potential source of bioactive secondary metabolites. In Lichen Secondary Metabolites; Ranković, B., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–26. [Google Scholar]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens-a potential source for nanoparticles fabrication: A review on nanoparticles biosynthesis and their prospective applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef]
- Suryanarayan, T.S.; Thirunavukkarasu, N.; Hariharan, G.N.; Balaji, P. Occurrence of non-obligate microfungi inside lichen thalli. Sydowia 2005, 57, 120–130. [Google Scholar]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar endophytes: Are tropical leaves biodiversity hotspots. Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Raja, H.A. Endolichenic fungi: A new source of rich bioactive secondary metabolites on the horizon. Phytochem. Rev. 2017, 16, 271–293. [Google Scholar] [CrossRef]
- Paranagama, P.A.; Wijeratne, E.K.; Burns, A.M.; Marron, M.T.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.L. Heptaketides from Corynespora sp. inhabiting the cavern beard lichen, Usnea cavernosa: First report of metabolites of an endolichenic fungus. J. Nat. Prod. 2007, 70, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zou, J.; Li, J.; Zhao, H. Endolichenic fungi: A potential treasure trove for discovery of special structures and bioactive compounds. Stud. Nat. Prod. Chem. 2016, 48, 347–397. [Google Scholar]
- Agrawal, S.; Deshmukh, S.K.; Reddy, M.S.; Prasad, R.; Goel, M. Endolichenic fungi: A hidden source of bioactive metabolites. S. Afr. J. Bot. 2020, 134, 163–168. [Google Scholar] [CrossRef]
- Yang, B.J.; Chen, G.D.; Li, Y.J.; Hu, D.; Guo, L.D.; Xiong, P.; Gao, H. A new xanthone glycoside from the endolichenic fungus Sporormiella irregularis. Molecules 2016, 21, 764. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Zhang, M.; Zhu, R.X.; Zhang, J.Z.; Xie, F.; Li, X.B.; Chang, W.Q.; Wang, X.N.; Zhao, Z.T.; Lou, H.X. Heptaketides from an endolichenic fungus Biatriospora sp. and their antifungal activity. J. Nat. Prod. 2016, 79, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Umeokoli, B.O.; Eze, P.; Heering, C.; Janiak, C.; Müller, W.E.; Orfali, R.S.; Hartmann, R.; Dai, H.; Lin, W.; et al. Secondary metabolites of the lichen-associated fungus Apiospora montagnei. Tetrahedron Lett. 2017, 58, 1702–1705. [Google Scholar] [CrossRef]
- Xie, F.; Luan, X.Y.; Gao, Y.; Xu, K.; Lou, H.X. Cytotoxic heptaketides from the endolichenic fungus Ulospora bilgramii. J. Nat. Prod. 2020, 83, 1623–1633. [Google Scholar] [CrossRef]
- Varlı, M.; Lee, E.-Y.; Yang, Y.; Zhou, R.; Taş, İ.; Pulat, S.; Gamage, C.D.B.; Park, S.-Y.; Hur, J.-S.; Nam, S.-J.; et al. 1′-O-Methyl-averantin isolated from the endolichenic fungus Jackrogersella sp. EL001672 suppresses colorectal cancer stemness via sonic hedgehog and notch signaling. Heliyon 2023, 13, 2811. [Google Scholar] [CrossRef]
- Padhi, S.; Masi, M.; Cimmino, A.; Tuzi, A.; Jena, S.; Tayung, K.; Evidente, A. Funiculosone, a substituted dihydroxanthene-1,9-dione with two of its analogues produced by an endolichenic fungus Talaromyces funiculosus and their antimicrobial activity. Phytochemistry 2019, 157, 175–183. [Google Scholar] [CrossRef]
- Lee, S.; Park, I.G.; Choi, J.W.; Son, J.Y.; Lee, J.W.; Hur, J.S.; Kim, Y.; Nam, S.J.; Kang, H.S.; Deyrup, S.T.; et al. Daldipyrenones A-C: Caged [6,6,6,6,6] polyketides derived from an endolichenic fungus Daldinia pyrenaica 047188. Org. Lett. 2023, 25, 6725–6729. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, W.; Kim, E.; Kim, G.S.; Hwang, G.J.; Son, S.; Jeong, M.-H.; Hur, J.-S.; Oh, H.; Ko, S.-K.; et al. Anti-inflammatory phomalichenones from an endolichenic fungus Phoma sp. J. Antibiot. 2018, 71, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, S.; Yan, S.; Zhang, M.; Cai, Q.; Fu, S.; Meng, Q. New phomalone derivatives from the endolichenic fungus Cochliobolus kusanoi in Ny-Alesund Arctic. J. Chin. Chem. Soc. 2018, 66, 325–329. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhu, R.-X.; Li, G.; Wang, N.-N.; Liu, C.-Y.; Zhao, Z.-T.; Lou, H.-X. Secondary metabolites from the endolichenic fungus Ophiosphaerella korrae. RSC Adv. 2019, 9, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.J.; Li, Y.J.; Tang, J.; Chen, G.D.; Hu, D.; Xu, W.; Guo, L.D.; Yao, X.S.; Gao, H. Spororrminone A and 2-epi-spororrminone A, two new chromones from an endolichenic fungus Sporormiella irregularis. Nat. Prod. Res. 2019, 34, 3117–3124. [Google Scholar] [CrossRef] [PubMed]
- Manthrirathna, M.; Kandiah, R.; Gunasekera, D.; Samanthi, K.; Welideniya, D.; Maduranga, H.; Paranagama, P. A secondary metabolite with in vitro radical scavenging activity from endolichenic fungus Daldinia eschscholzii found in lichen, Parmotrema sp. in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2020, 48, 143–148. [Google Scholar] [CrossRef]
- Zhang, D.; Gu, G.; Zhang, B.; Wang, Y.; Bai, J.; Fang, Y.; Zhang, T.; Dai, S.; Cen, S.; Yu, L. New phenol and chromone derivatives from the endolichenic fungus Daldinia species and their antiviral activities. RSC Adv. 2021, 11, 22489–22494. [Google Scholar] [CrossRef] [PubMed]
- Shevkar, C.; Armarkar, A.; Weerasinghe, R.; Maduranga, K.; Pandey, K.; Behera, S.K.; Kalia, K.; Paranagama, P.; Kate, A.S. Cytotoxic bioxanthracene and macrocyclic polyester from endolichenic fungus Talaromyces pinophilus: In-vitro and in-silico analysis. Indian J. Microbiol. 2022, 62, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.-H.; Teng, M.-T.; Yun, Y.-F.; Jiang, N.; Ma, L.; Sun, S.-S.; Yuan, B.; Tang, J.; Wu, Q.-Y.; Li, Q.; et al. Talarolactone A, an isocoumarin derivative fused with dihydrothiophene with selective antimigratory activity from the endolichenic fungus Talaromyces sp. J. Nat. Prod. 2020, 83, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-Z.; Su, B.-J.; Chen, Y.-C.; Xiao, T.-M.; Yan, B.-Y.; Yu, L.-Y.; Si, S.-Y.; Wu, D.-L.; Chen, M.-H. Three new isocoumarin analogues from an endolichenic fungus Aspergillus flavus CPCC 400810. Nat. Prod. Res. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Samanthi, K.; Wickramarachchi, S.; Wijeratne, E.; Paranagama, P. Two new bioactive polyketides from Curvularia trifolii, an endolichenic fungus isolated from Usnea sp., in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2015, 433, 217–224. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, Y.-H.; Wang, H.-Y.; Ma, X.-J.; Jiang, T.; Zhao, J.-L.; Zou, Z.-M.; Ding, G. Allelopathic polyketides from an endolichenic fungus Myxotrichum sp. by using OSMAC strategy. Sci. Rep. 2016, 6, 19350. [Google Scholar] [CrossRef]
- Hu, C.H.; Zhou, Y.H.; Xie, F.; Li, Y.L.; Zhao, Z.T.; Lou, H.X. Two new α-pyrone derivatives from an endolichenic fungus Tolypocladium sp. J. Asian Nat. Prod. Res. 2017, 19, 786–792. [Google Scholar] [CrossRef]
- Padhi, S.; Masi, M.; Panda, S.K.; Luyten, W.; Cimmino, A.; Tayung, K.; Evidente, A. Antimicrobial secondary metabolites of an endolichenic Aspergillus niger isolated from lichen thallus of Parmotrema ravum. Nat. Prod. Res. 2019, 34, 2573–2580. [Google Scholar] [CrossRef]
- Kim, Y.J.; Duraisamy, K.; Jeong, M.-H.; Park, S.-Y.; Kim, S.; Lee, Y.; Nguyen, V.T.; Yu, N.H.; Park, A.R.; Kim, J.-C. Nematicidal activity of grammicin biosynthesis pathway intermediates in Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Molecules 2021, 26, 4675. [Google Scholar] [CrossRef]
- Lin, L.-B.; Gao, Y.-Q.; Han, R.; Xiao, J.; Wang, Y.-M.; Zhang, Q.; Zhai, Y.-J.; Han, W.-B.; Li, W.-L.; Gao, J.-M. Alkylated salicylaldehydes and prenylated indole alkaloids from the endolichenic fungus Aspergillus chevalieri and their bioactivities. J. Agric. Food Chem. 2021, 69, 6524–6534. [Google Scholar] [CrossRef]
- Gamage, C.D.; Lee, K.; Park, S.Y.; Varlı, M.; Lee, C.W.; Kim, S.M.; Zhou, R.; Pulat, S.; Yang, Y.; Taş, İ.; et al. Phthalides isolated from the endolichenic Arthrinium sp. EL000127 exhibits antiangiogenic activity. ACS Omega 2023, 8, 12548–12557. [Google Scholar] [CrossRef]
- Maduranga, H.; Weerasinghe, W.; Attanayake; Santhirasegaram, S.; Shevkar, C.; Kate, A.; Weerakoon, G.; Samanthi, K.; Kalia, K.; Paranagama, P.A. Identification of novel bioactive compounds, neurosporalol 1 and 2 from an endolichenic fungus, neurospora ugadawe inhabited in the lichen host, Graphis tsunodae Zahlbr. from mangrove ecosystem in Puttalam Lagoon, Sri Lanka. Asian J. Chem. 2021, 33, 1425–1432. [Google Scholar] [CrossRef]
- Kim, J.; He, M.T.; Hur, J.-S.; Lee, J.W.; Bin Kang, K.; Kang, K.S.; Shim, S.H. Discovery of naphthol tetramers from endolichenic fungus Daldinia childiae 047219 based on MS/MS molecular networking. J. Nat. Prod. 2023, 86, 2031–2038. [Google Scholar] [CrossRef]
- Wijeratne, E.K.; Gunaherath GK, B.; Chapla, V.M.; Tillotson, J.; De La Cruz, F.; Kang, M.; U’Ren, J.M.; Araujo, A.R.; Arnold, A.E.; Chapman, E.; et al. Oxaspirol B with p97 inhibitory activity and other oxaspirols from Lecythophora sp. FL1375 and FL1031, endolichenic fungi inhabiting Parmotrema tinctorum and Cladonia evansii. J. Nat. Prod. 2016, 79, 340–352. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jang, J.Y.; Yu, N.H.; Chi, W.J.; Bae, C.; Yeo, J.H.; Park, A.R.; Hur, J.; Park, H.W.; Park, J.; et al. Nematicidal activity of grammicin produced by Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Pest Manag. Sci. 2017, 74, 384–391. [Google Scholar] [CrossRef]
- Ma, J.; Cao, B.; Liu, C.; Guan, P.; Mu, Y.; Jiang, Y.; Han, L.; Huang, X. Actinofuranones D-I from a lichen-associated actinomycetes, Streptomyces gramineus, and their anti-inflammatory effects. Molecules 2018, 23, 2393. [Google Scholar] [CrossRef]
- Basnet, B.B.; Chen, B.; Suleimen, Y.M.; Ma, K.; Guo, S.; Bao, L.; Huang, Y.; Liu, H. Cytotoxic secondary metabolites from the endolichenic fungus Hypoxylon fuscum. Planta Med. 2019, 85, 1088–1097. [Google Scholar] [CrossRef]
- Santhirasegaram, S.; Wickramarachchi, S.R.; Attanayake, R.N.; Weerakoon, G.; Samarakoon, S.; Wijeratne, K.; Paranagama, P.A. A novel cytotoxic compound from the endolichenic fungus, Xylaria psidii inhabiting the lichen, Amandinea medusulina. Nat. Prod. Commun. 2020, 15, 1700–1705. [Google Scholar] [CrossRef]
- Xu, K.; Li, G.; Zhu, R.; Xie, F.; Li, Y.; Yang, W.; Xu, L.; Shen, T.; Zhao, Z.; Lou, H. Polyketides from the endolichenic fungus Eupenicillium javanicum and their anti-inflammatory activities. Phytochemistry 2020, 170, 112191. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.-Y.; Xie, F.; Xu, K.; Gao, Y.; Lu, J.-H.; Lou, H.-X. (±)-Ulodione A, a pair of unprecedented cyclopentanones from Ulospora bilgramii. Tetrahedron Lett. 2020, 61, 151732. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.X.; Yu, Y.; Wang, G.Q.; Zheng, Q.C.; Chen, G.D.; Lian, Y.Y.; Lin, F.; Guo, L.D.; Gao, H. Nodulisporipyrones A-D, new bioactive α-pyrone derivatives from Nodulis poriumsp. J. Asian Nat. Prod. Res. 2015, 17, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.-B.; Li, L.; Li, R.-J.; Lou, H.-X. α-Pyrone derivatives from the endolichenic fungus Nectria sp. Phytochem. Lett. 2015, 12, 22–26. [Google Scholar] [CrossRef]
- Kim, G.S.; Ko, W.; Kim, J.W.; Jeong, M.H.; Ko, S.K.; Hur, J.S.; Oh, H.; Jang, J.H.; Ahn, J.S. Bioactive α-pyrone derivatives from the endolichenic fungus Dothideomycetes sp. EL003334. J. Nat. Prod. 2018, 81, 1084–1088. [Google Scholar] [CrossRef]
- Aursnes, M.; Primdahl, K.G.; Liwara, D.; Solum, E.J. A modular strategy for the synthesis of dothideopyrones E and F, secondary metabolites from an endolichenic fungus. J. Nat. Prod. 2023, 86, 804–811. [Google Scholar] [CrossRef]
- Yuan, W.; Teng, M.; Sun, S.; Ma, L.; Yuan, B.; Ren, Q.; Zhang, P. Active metabolites from endolichenic fungus Talaromyces sp. Chem. Biodivers. 2018, 15, e1800371. [Google Scholar] [CrossRef]
- Yuan, C.; Ding, G.; Wang, H.-Y.; Guo, Y.-H.; Shang, H.; Ma, X.-J.; Zou, Z.-M. Polyketide-terpene hybrid metabolites from an endolichenic fungus Pestalotiopsis sp. BioMed Res. Int. 2017, 2017, 6961928. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Ding, G.; Wang, H.; Guo, Y.; Ma, X.; Zou, Z. Phytotoxic secondary metabolites from the endolichenic fungus Myxotrichum sp. Chem. Nat. Compd. 2018, 54, 638–641. [Google Scholar] [CrossRef]
- Cai, Y.-S.; Guo, Y.-W.; Krohn, K. Structure, bioactivities, biosynthetic relationships and chemical synthesis of the spirodioxynaphthalenes. Nat. Prod. Rep. 2010, 27, 1840–1870. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chang, W.; Zhang, M.; Li, Y.; Li, W.; Shi, H.; Zheng, S.; Lou, H. Quinone derivatives isolated from the endolichenic fungus Phialocephala fortinii are Mdr1 modulators that combat azole resistance in Candida albicans. Sci. Rep. 2016, 6, 33687. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, R.; Gao, H.; Guo, L.; Yao, X.; Zhang, Y.; Tang, J. New spirobisnaphthalenes from an endolichenic fungus strain CGMCC 3.15192 and their anticancer effects through the P53-P21 pathway. RSC Adv. 2019, 9, 39082–39089. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xie, F.; Luan, X.; Xu, K.; Qian, L.; Lu, J.; Chang, W.; Wang, X.; Lou, H. Perylenequinone derivatives from the endolichenic fungus Phialocephala fortinii. Nat. Prod. Res. 2022, 37, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.-J.; Zhou, Z.-Z.; Gao, L.-L.; Li, J.-N.; Pescitelli, G.; Gao, J.-M.; Han, W.-B. Ethylidene-tethered chromene-pyrone hybrids as potential plant-growth regulators from an endolichenic Phaeosphaeria species. J. Agric. Food Chem. 2023, 71, 4615–4624. [Google Scholar] [CrossRef]
- Li, W.; Gao, W.; Zhang, M.; Li, Y.-L.; Li, L.; Li, X.-B.; Chang, W.-Q.; Zhao, Z.-T.; Lou, H.-X. p-Terphenyl derivatives from the endolichenic fungus Floricola striata. J. Nat. Prod. 2016, 79, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, Y.; Li, Y.-L.; Xie, F.; Zhao, Z.-T.; Lou, H.-X. Cytotoxic p-terphenyls from the endolichenic fungus Floricola striata. J. Nat. Prod. 2018, 81, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Knaggs, A.R. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 2003, 20, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Gill, M. Pigments of fungi (Macromycetes). Nat. Prod. Rep. 2003, 20, 615–639. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chen, G.D.; Wang, C.X.; Hu, D.; Li, X.X.; Lian, Y.Y.; Lin, F.; Guo, L.D.; Gao, H. Pericoterpenoid A, a new bioactive cadinane-type sesquiterpene from Periconia sp. J. Asian Nat. Prod. Res. 2015, 17, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, C.; Meng, Q.; Liu, L.; Fu, S. A new alkanol from the endolichenic fungus Daldinia childiae. J. Chin. Chem. Soc. 2020, 68, 678–681. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Li, J.-N.; Gao, Y.-Q.; Gao, L.-L.; Wang, D.-C.; Han, W.-B.; Gao, J.-M. Structurally diverse sesquiterpenoids with anti-neuroinflammatory activity from the endolichenic fungus Cryptomarasmius aucubae. Nat. Prod. Bioprospect. 2021, 11, 325–332. [Google Scholar] [CrossRef]
- Miral, A.; Ferron, S.; Rouaud, I.; Slyambayev, D.; Bousarghin, L.; Camuzet, C.; Belouzard, S.; Séron, K.; Le Pogam, P.; Tranchimand, S.; et al. Eremoxylarins D-J, antibacterial eremophilane sesquiterpenes discovered from an endolichenic strain of Xylaria hypoxylon. J. Nat. Prod. 2023, 86, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, Y.; Zhu, S.; Zhang, Y.; Guo, L.; Qiu, F.; Che, Y. Sarcosenones A-C, highly oxygenated pimarane diterpenoids from an endolichenic fungus Sarcosomataceae sp. RSC Adv. 2020, 10, 15622–15628. [Google Scholar] [CrossRef]
- Basnet, B.B.; Liu, L.; Chen, B.; Suleimen, Y.M.; Yu, H.; Guo, S.; Bao, L.; Ren, J.; Liu, H. Four new cytotoxic arborinane-type triterpenes from the endolichenic fungus Myrothecium inundatum. Planta Med. 2019, 85, 701–707. [Google Scholar] [CrossRef]
- Varlı, M.; Pham, H.T.; Kim, S.-M.; Taş, İ.; Gamage, C.D.B.; Zhou, R.; Pulat, S.; Park, S.-Y.; Sesal, N.C.; Hur, J.-S.; et al. An acetonic extract and secondary metabolites from the endolichenic fungus Nemania sp. EL006872 exhibit immune checkpoint inhibitory activity in lung cancer cell. Front. Pharmacol. 2022, 13, 986946. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-X.; Li, Y.-Y.; Qian, X.-M.; Shen, Y.-M. Guanacastane-type diterpenoids from Coprinus radians. Phytochemistry 2012, 78, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gamage, C.D.B.; Kim, J.-H.; Yang, Y.; Taş, İ.; Park, S.-Y.; Zhou, R.; Pulat, S.; Varlı, M.; Hur, J.-S.; Nam, S.-J.; et al. Libertellenone T, a novel compound isolated from endolichenic fungus, induces G2/M phase arrest, apoptosis, and autophagy by activating the ROS/JNK pathway in colorectal cancer cells. Cancers 2023, 15, 489. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Gao, Y.; Liu, C.Y.; Sun, C.J.; Zhao, Z.T.; Lou, H.X. Asperunguisins A-F, cytotoxic asperane sesterterpenoids from the endolichenic fungus Aspergillus unguis. J. Nat. Prod. 2019, 82, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tamayo-Castillo, G.; Pang, C.; Clardy, J.; Cao, S.; Kim, K.H. Diketopiperazines from Costa Rican endolichenic fungus Colpoma sp. CR1465A. Bioorg. Med. Chem. Lett. 2016, 26, 2438–2441. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, R.; Zhao, W.; Yu, L.; Zhang, C.; Chang, S.; Li, Y.; Zhang, T.; Xing, J.; Gan, M.; et al. Isocoumarindole A, a chlorinated isocoumarin and indole alkaloid hybrid metabolite from an endolichenic fungus Aspergillus sp. Org. Lett. 2019, 21, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, R.; Zhu, R.; Li, X.; Xu, Y.; He, Q.; Xie, F.; Qiao, Y.; Luan, X.; Lou, H. Xylarins A-D, two pairs of diastereoisomeric isoindoline alkaloids from the endolichenic fungus Xylaria sp. Org. Lett. 2021, 23, 7751–7754. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dai, H.; Bao, L.; Ren, B.; Lu, J.; Luo, Y.; Guo, L.; Zhang, L.; Liu, H. Isolation and structural elucidation of proline-containing cyclopentapeptides from an endolichenic Xylaria sp. J. Nat. Prod. 2011, 74, 1303–1308. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Hu, K.; Wang, F.; Tang, J.-W.; Zhang, L.; Sun, H.-D.; Cai, X.-H.; Puno, P.-T. 3-Hydroxy-4-methyldecanoic acid-containing cyclotetradepsipeptides from an endolichenic Beauveria sp. J. Nat. Prod. 2021, 84, 1244–1253. [Google Scholar] [CrossRef]
- Luo, M.; Chang, S.; Li, Y.; Xi, X.; Chen, M.; He, N.; Wang, M.; Zhao, W.; Xie, Y. Molecular networking-based screening led to the discovery of a cyclic heptadepsipeptide from an endolichenic Xylaria sp. J. Nat. Prod. 2022, 85, 972–979. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, G.D.; Feng, X.L.; Yu, Y.; He, R.R.; Li, X.X.; Huang, Y.; Zhou, W.X.; Guo, L.D.; Zheng, Y.Z.; et al. Nodulisporiviridins A-H, bioactive viridins from Nodulisporium sp. J. Nat. Prod. 2015, 78, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, G.-Q.; Chen, G.-D.; Hu, D.; Li, X.-X.; Guo, L.-D.; Li, Y.; Yao, X.-S.; Gao, H. Nodulisporisteroids C-L, new 4-methyl-progesteroid derivatives from Nodulisporium sp. Steroids 2015, 102, 101–109. [Google Scholar] [CrossRef]
- Brown, G.D. The biosynthesis of steroids and triterpenoids. Nat. Prod. Rep. 1998, 15, 653–696. [Google Scholar] [CrossRef]
- Tan, M.A.; Castro, S.G.; Oliva, P.M.P.; Yap, P.R.J.; Nakayama, A.; Magpantay, H.D.; Cruz, T.E.E.D. Biodiscovery of antibacterial constituents from the endolichenic fungi isolated from Parmotrema rampoddense. 3 Biotech 2020, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Toure, S.; Millot, M.; Ory, L.; Roullier, C.; Khaldi, Z.; Pichon, V.; Girardot, M.; Imbert, C.; Mambu, L. Access to anti-biofilm bompounds from endolichenic fungi using a bioguided networking screening. J. Fungi 2022, 8, 1012. [Google Scholar] [CrossRef] [PubMed]
- Varlı, M.; Ngo, M.T.; Kim, S.-M.; Taş, İ.; Zhou, R.; Gamage, C.D.; Pulat, S.; Park, S.-Y.; Sesal, N.C.; Hur, J.-S.; et al. A fatty acid-rich fraction of an endolichenic fungus Phoma sp. suppresses immune checkpoint markers via AhR/ARNT and ESR1. Heliyon 2023, 9, e19185. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Suzuki, C.; Yamaguchi, H.; Hara, K.; Komine, M.; Yamamoto, Y. Norlichexanthone produced by cultured endolichenic fungus induced from Pertusaria laeviganda and its antioxidant activity. Biosci. Biotechnol. Biochem. 2019, 83, 996–999. [Google Scholar] [CrossRef] [PubMed]
































| Type of Compound | Compound | Cell Line | IC50 | Endolichenic Fungus | Host | Reference |
|---|---|---|---|---|---|---|
| Polyketides | bostrycin (16) | L5178 | 1.7 μM | A. montagnei | Cladonia sp. | Wang et al., 2017 [23] |
| Polyketides | ulosporins G (23) | A549 | 1.3 μM | U. bilgramii | Umbilicaria sp. | Xie et al., 2020 [24] |
| MCF-7 | 1.3 μM | |||||
| KB | 3.0 μM | |||||
| Polyketides | 1′-O-methyl-averantin (29) | CSC221 | 19.34 µg/mL | Jackrogersella sp. | Cetraria sp. | Varlı et al., 2023 [25] |
| Caco2 | 18.35 µg/mL | |||||
| DLD1 | 21.50 µg/mL | |||||
| HCT116 | 22.78 µg/mL | |||||
| Polyketides | acremonone G (67) | L5178 | 2.7 μM | A. montagnei | Cladonia sp. | Wang et al., 2017 [23] |
| Polyketides | 3-O-phenylethyl cyclopolic acid (115) | HUVEC | 43.8 μM | Arthrinium sp. | Cladonia squamosal | Gamage et al., 2023 [43] |
| Polyketides | 3-O-p-hydroxy phenyl ethylcyclo polic acid (116) | HUVEC | 1.83 mM | Arthrinium sp. | C. squamosal | Gamage et al., 2023 [43] |
| Polyketides | hypoxyolide A (138) | K562, SW480, HEPG2 | 12.0–32.7 µM | H. fuscum | Usnea sp. | Basnet et al., 2019 [49] |
| Polyketides | alaromycin A (170) | THLE | 46.6 ± 3.8 μM | Talaromyces sp. | X. angustiphylla | Yuan et al., 2018 [57] |
| MDA-MB-231 | 24.6 ± 1.3 μM | |||||
| Polyketides | clearanol A (171) | THLE | 51.6 ± 3.2 μM | Talaromyces sp. | X. angustiphylla | Yuan et al., 2018 [57] |
| MDA-MB-231 | 19.1 ± 1.2 μM | |||||
| Polyketides | phomol (201) | K562 | 19.4 μM | H. fuscum | Usnea sp. | Basnet et al., 2019 [49] |
| SW480 | 15.9 μM | |||||
| HEPG2 | 32.7 μM | |||||
| Polyketides | 5,6-epoxy-phomol (202) | K562 | 12.0–32.7 µM | H. fuscum | Usnea sp. | Basnet et al., 2019 [49] |
| SW480 | ||||||
| HEPG2 | ||||||
| Polyketides | palmarumycin P3 (209) | EC109 | 24.5–33.3 μM | P. fortinii | Pamelia sp. | Song e al. 2023 [63] |
| Polyketides | plecmillin A (218) | HCT116 | 2.1 μM | / | Peltigera elisabethae | Li et al., 2019 [62] |
| Polyketides | phialoce-phalarin H (220) | EC109 | 24.5–33.3 μM | P. fortinii | Pamelia sp. | Song e al. 2023 [63] |
| Polyketides | phialoce-phalarin I (221) | EC109 | 24.5–33.3 μM | P. fortinii | Pamelia sp. | Song e al. 2023 [63] |
| Polyketides | phialoce-phalarin A (225) | EC109 | 24.5–33.3 μM | P. fortinii | Pamelia sp. | Song e al. 2023 [63] |
| Polyphenyls | Floricolin K (275) | A2780 | 25.1 ± 2.3 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| MCF-7 | 13.4 ± 1.4 μM | |||||
| Polyphenyls | Floricolin L (276) | A2780 | 21.4 ± 1.9 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| MCF-7 | 17.9 ± 2.4 μM | |||||
| Polyphenyls | Floricolin M (277) | A2780 | 40.1 ± 3.8 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| MCF-7 | 17.5 ± 1.7 μM | |||||
| Polyphenyls | Floricolin N (278) | A2780 | 14.9 ± 1.4 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| MCF-7 | 11.7 ± 1.2 μM | |||||
| A549 | 27.8 ± 2.0 μM | |||||
| Polyphenyls | Floricolin O (279) | A2780 | 3.4 ± 0.6 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| MCF-7 | 19.9 ± 2.1 μM | |||||
| A549 | 40.1 ± 3.8 μM | |||||
| Polyphenyls | Floricolin P (280) | A2780 | 8.6 ± 1.0 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| MCF-7 | 16.7 ± 0.8 μM | |||||
| Polyphenyls | Floricolin T (284) | MCF-7 | 38.5 ± 4.0 μM | F. striata | Pseudosyphellaria spp. | Xu et al., 2018 [66] |
| A549 | 12.5 ± 2.5 μM | |||||
| Polyphenyls | 6′-methyl-[1,1′-biphenyl]-3,3′,4′,5-tetraol (292) | HBE | 29.3 ±1.7 μM | Talaromyces sp. | X. angustiphylla | Yuan et al., 2018 [57] |
| THLE | 36.2 ±1.9 μM | |||||
| MDA-MB-231 | 34.7 ±4.4 μM | |||||
| Polyphenyls | desmethylaltenusin (293) | HBE | 43.9 ± 1.6 μM | Talaromyces sp. | X. angustiphylla | Yuan et al., 2018 [57] |
| THLE | 41.2 ± 6.4 μM | |||||
| Polyketides | ES-242-3 (250) | MCF-7 | 14.08 ± 0.2 μM | T. pinophilus | Porina tetracerae | Shevkar et al., 2022 [34] |
| HeLa cell line | 4.46 ± 0.05 μM | |||||
| Terpenoids | hypoxyside A (296) | K562 | 18.7 μM | H.fuscum | Usnea sp. | Basnet et al., 2019 [49] |
| Terpenoids | eremoxylarin I (311) | HCoV-229E | 18.1 μM | X. hypoxylon | Rhizocarpon geographicum | Miral et al., 2023 [72] |
| Terpenoids | sarcosenones A (313) | MCF-7 | 10.3 ± 1.0 μM | Sarcosomataceae sp. | Everniastrum sp. | Hou et al., 2019 [73] |
| HeLa | 11.9 ± 4.4 μM | |||||
| HepG2 | 26.4 ± 3.2 μM | |||||
| 786-O | 26.4 ± 3.2 μM | |||||
| Terpenoids | sphaeropsidin A (317) | K562 | 28.6 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| Terpenoids | hymatoxin L (318) | RKO | 68.8 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| K562 | 32.5 μM | |||||
| Terpenoids | 16-α-D-mannopyranosyl- oxyisopimar-7-en-19-oic acid (319) | RKO | 31.7 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| K562 | 7.60 μM | |||||
| Terpenoids | 16-α-D-glucopyranosyl- oxyisopimar-7-en-19-oic acid (320) | K562 | 27.4 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| Terpenoids | radianspene C (321) | MDA-MB-435 | 0.91 μM | Nemania sp. | Bryoria fuscescens | Varlı et al., 2022 [75] |
| Polyketides | libertellenone T (324) | Caco2 | 17.5 µg/mL | Pseudoplectania sp. | Specimen Graphis | Gamage et al., 2023 [77] |
| HCT116 | 28 µg/mL | |||||
| DLD1 | 36.6 µg/mL | |||||
| HT29 | 28 µg/mL | |||||
| Terpenoids | myrotheol A (333) | RKO | 51.0 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| K562 | 28 μM | |||||
| Terpenoids | myrotheol B (334) | RKO | 51.0 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| K562 | 28 μM | |||||
| Terpenoids | myrotheside D (336) | RKO | 62.3 μM | M. inundatum | Ramalina sp. | Basnet et al., 2019 [74] |
| K562 | 63.9 μM | |||||
| Alkaloids | isocoumarindole A (345) | MIA-PaCa-2 | 1.63 μM | Aspergillus sp. | Cetrelia sp. | Chen et al., 2019 [80] |
| AsPC-1 | 5.53 μM | |||||
| Alkaloids | (Z)-3-{(3-acetyl-2- hydroxyphenyl)diazenyl}-2,4-dihydroxybenzaldehyde (347) | NCI-H292 | 27.2 µg/mL | X. psidii | Amandinea medusulina | Santhirasegaram et al., 2020 [50] |
| Alkaloids | xylaroamide A (370) | BT-549 | 2.5 μM | Xylaria sp. | Usnea sp. | Luo et al., 2022 [84] |
| RKO | 9.5 μM | |||||
| Steroids | ergone (389) | MDA-MB-231 | 33 ± 0.5 μM 24.9 ± 3.7 μM 20.3 ± 4.4 μM | Talaromyces sp. | X. angustiphylla | Yuan et al., 2018 [57] |
| ergosterol (390) | MDA-MB-231 | |||||
| Fatty acid | palmitic acid (399) | THLE | 17.1 ± 0.7 μM | Talaromyces sp. | X. angustiphylla | Yuan et al., 2018 [57] |
| HBE | 17.2 ± 0.5 μM | |||||
| MDA-MB-231 | 28.6 ± 3.2 μM |
| Type of Compound | Compound | Microbe | MIC/IC50 | Endolichenic Fungus | Host | Reference |
|---|---|---|---|---|---|---|
| Polyketides | funiculosone (38) | S. aureus | 25 μg/mL | T. funiculosus | Diorygma hieroglyphicum | Padhi et al., 2019 [26] |
| E. coli | 58 μg/mL | |||||
| Candida albicans | 35 μg/mL | |||||
| Polyketides | mangrovamide J (39) | S. aureus, E. coli | 23–104 μg/mL | T. funiculosus | D. hieroglyphicum | Padhi et al., 2019 [26] |
| Polyketides | ravenelin (40) | S. aureus, E. coli | 23–104 μg/mL | T. funiculosus | D. hieroglyphicum | Padhi et al., 2019 [26] |
| Polyketides | asperglaucins B (106) | P. syringae pv actinidae. Bacillus cereus. | 6.25 μM | A. chevalieri | L. incana | Lin et al., 2021 [42] |
| Polyketides | phomol (201) | S. aureus | 51.2 µM | H. fuscum | Usnea sp. | Basnet et al., 2019 [49] |
| Polyphenyls | floricolin A (259) | C. albicans | 16 μg/mL | F. striata | Umbilicaria sp. | Li et al., 2016 [65] |
| Polyphenyls | floricolin B (260) | C. albicans | 8 μg/mL | F. striata | Umbilicaria sp. | Li et al., 2016 [65] |
| Polyphenyls | floricolin C (261) | C. albicans | 8 μg/mL | F. striata | Umbilicaria sp. | Li et al., 2016 [65] |
| Terpenoids | eremoxylarins D (306) | S.aureus | 6.25 μg/mL | X. hypoxylon | R. geographicum | Miral et al., 2023 [72] |
| Terpenoids | eremoxylarins D (306) | MRSA | 12.5 μg/mL | X.hypoxylon | R. geographicum | Miral et al., 2023 [72] |
| S. epidermidis. | 12.5 μg/mL | |||||
| Terpenoids | eremoxylarins F (308) | S. aureus | 0.78 μg/mL | X. hypoxylon | R. geographicum | Miral et al., 2023 [72] |
| MRSA | 1.56 μg/mL | |||||
| S. epidermidis. | 3.10 μg/mL | |||||
| Terpenoids | eremoxylarins G (309) | S. aureus | 1.56 μg/mL | X. hypoxylon | R. geographicum | Miral et al., 2023 [72] |
| MRSA | 3.10 μg/mL | |||||
| S. epidermidis. | 3.10 μg/mL | |||||
| Terpenoids | eremoxylarins I (311) | S. aureus | 0.39 μg/mL | X. hypoxylon | R. geographicum | Miral et al., 2023 [72] |
| MRSA | 1.56 μg/mL | |||||
| S. epidermidis. | 1.56 μg/mL | |||||
| Terpenoids | 16-α-D-mannopyranosyl-oxyisopimar-7-en-19-oic acid (319) | S. aureus | 96.5 µM | H. fuscum | Usnea sp. | Basnet et al., 2019 [49] |
| Alkaloids | Isocoumarindole A (345) | C. albicans | 32.0 μg/mL | Aspergillus sp. | Cetrelia sp. | Chen et al., 2019 [80] |
| others | acetyl tributyl citrate (396) | K. pneumoniae | 3.11 μM | F. proliferatum | Parmotrema rampoddense | Tan et al., 2020 [88] |
| P. aeruginosa | 0.19 μM | |||||
| S. aureus | 0.78 μM |
| Type of Compound | Compound | Biological Target | Biological Active Value (MIC/IC50) | Endolichenic Fungus | Host | Reference |
|---|---|---|---|---|---|---|
| Polyketides | norlichexanthone (34) | DDPH assay | ORAC value (mol TE/g) of 0.0202 | Dothideomycetes sp. | Pertusaria laeviganda | Kawakami et al., 2019 [91] |
| Polyketides | 5-methoxy-4,8,15-trimethyl-3,7-dioxo-1,3,7,8,9,10,11,12,13, 14,15,15α-dodecahydrocycl-ododeca[de]isochromene-15-carboxylic acid (80) | DDPH assay | 1.3 ± 0.2 mg/mL | C. trifolii | Usnea sp. | Samanthi et al., 2015 [37] |
| Polyketides | 8-methoxynaphthalen-1-ol (96) | DDPH assay | 10.2 ± 5.8 µg/mL | D. eschscholzii | Parmotrema sp. | Manthrirathna et al., 2020 [32] |
| Polyketides | tetrahydroauroglaucin (107) | DDPH assay | 11.0 ± 0.2 μM | A. chevalieri | L. incana | Lin et al., 2021 [42] |
| Polyketides | flavoglaucin (108) | DDPH assay | 11.5 ± 0.6 μM | A. chevalieri | L. incana | Lin et al., 2021 [42] |
| Polyketides | 2-(1′,5′-heptadienyl)-3,6-dihydroxy-5-(3″-methyl-2″-butenyl) benzaldehyde (109) | DDPH assay | 12.3 ± 0.5 μM | A. chevalieri | L. incana | Lin et al., 2021 [42] |
| Polyketides | 2-(E-3-heptenyl)-3,6-dihydroxy-5-(3-methyl-2-butenyl)-benzalde-hyde (111) | DDPH assay | 10.6 ± 0.1 μM | A. chevalieri | L. incana | Lin et al., 2021 [42] |
| Polyketides | neurosporalol 1 (117) | ABTS assay | 3.48 ± 0.33 µg/mL | N. ugadawe | G. tsunodae | Madyranga et al., 2021 [44] |
| Polyketides | 1,14-dihydroxy-6-methyl-6,7,8,9,10,10α,14,14α-octahydro-1H-benzo [f][1] oxacyclododecin-4(13H)-one(203) | DDPH assay | 4.0 ± 2.6 mg/mL | C. trifolii | Usnea sp. | Samanthi et al., 2015 [37] |
| Polyketides | neurosporalol 2 206 | ABTS assay | 5.03 ± 0.15 µg/mL | N. ugadawe | G. tsunodae | Madyranga et al., 2021 [44] |
| Type of Compound | Compound | Biological Target | Biological Actve Value (MIC/IC50) | Endolichenic Fungus | Host | Reference |
|---|---|---|---|---|---|---|
| Polyketides | phomalichenone A (84) | inhibition of NO production | 9.4 ± 0.5 μM | Phoma sp. | / | Kim et al., 2018 [28] |
| Polyketides | (E)-1-(2,4-dihydroxy-3-(2-hydroxyethyl)-6-methoxyphenyl)but-2-en-1-one (87) | inhibition of NO production | 7.4 ± 2.8 μM | Phoma sp. | / | Kim et al., 2018 [28] |
| Polyketides | javanicol E (146) | inhibition of NO production | 17.00 μM | E. javanicum | Parmelia sp. | Xu et al., 2020 [51] |
| Polyketides | (+)-terrein (147) | inhibition of NO production | 13.46 μM | E. javanicum | Parmelia sp. | Xu et al., 2020 [51] |
| Polyketides | dothideopyrone F (169) | inhibition of NO production | 15.0 ± 2.8 μM | Dothideomycetes sp. | Stereocaulon tomentosum | Kim et al., 2018 [55] |
| Polyketides | nesurosporalol 2 (206) | HRBCM stabilization assay | 129.03 ± 0.15 µg/mL | N. ugadawe | G tsunodae | Madyranga et al., 2021 [44] |
| Terpenoids | sterpurol D (299) | inhibition of NO production | 14.81 ± 2.23 μM | C. aucubae | / | Zhai et al., 2021 [71] |
| Terpenoids | sterpurol E (300) | inhibition of NO production | 9.93 ± 0.99 μM | C. aucubae | / | Zhai et al., 2021 [71] |
| Terpenoids | sterpurol A (302) | inhibition of NO production | 15.32 ± 1.43 μM | C. aucubae | / | Zhai et al., 2021 [71] |
| Terpenoids | sterpurol B (303) | inhibition of NO production | 9.06 ± 1.13 μM | C. aucubae | / | Zhai et al., 2021 [71] |
| Terpenoids | paneolilludinic acid (304) | inhibition of NO production | 11.49 ± 0.58 μM | C. aucubae | / | Zhai et al., 2021 [71] |
| Alkaloids | neoechinulin C (354) | inhibition of NO production | 12.0 μM | A. chevalieri | L. incana | Lin et al., 2021 [42] |
| Fatty acids | (–)-10,11-dihydroxyfarnesol (397) | inhibition of NO production | 12.17 ± 0.40 μM | C. aucubae | / | Zhai et al., 2021 [71] |
| Type of Compound | Compound | Activity | Biological Active Value (MIC/IC50) | Endolichenic Fungus | Host | Reference |
|---|---|---|---|---|---|---|
| Polyketides | daldipyrenone A (41) | adinonectu-secretion promoting activity | 3.36 μM | D. pyrenaica | Myelochroa aurulenta | Lee et al., 2023 [27] |
| Polyketides | (5R,7R)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (54) | anti-influenza A virus | 16.1 mM | Daldinia sp. | / | Zhang et al., 2021 [33] |
| Polyketides | (5R,7S)-5,7-dihydroxy-2-methyl-5,6,7,8-tetrahydro-4H-chromen-4-one (56) | anti-influenza A virus | 9.0 mM | Daldinia sp. | / | Zhang et al., 2021 [33] |
| Polyketides | daldispol A (97) | anti-influenza A virus | 12.7 mM | Daldinia sp. | / | Zhang et al., 2021 [33] |
| Polyketides | daldispol C (98) | anti-influenza A virus | 6.4 mM | Daldinia sp. | / | Zhang et al., 2021 [33] |
| Polyketides | 2-phenylethyl-β-D-glucopyranoside (100) | anti-influenza A virus | 12.5 mM | Daldinia sp. | / | Zhang et al., 2021 [33] |
| Polyketides | p-hydroxyphenethyl alcohol (104) | anti-ZIKV activity | inhibitory ratio of 42.7% at 10 μM | Daldinia sp. | / | Zhang et al., 2021 [33] |
| Polyketides | (R)-ulodione A(149a) | anti-butyrylcholinesterase | 9.0 ± 0.1μM | U. bilgramii | Umbilicaria sp. | Luan et al., 2020 [52] |
| Polyketides | (S)-ulodione A(149b) | anti-butyrylcholinesterase | 9.3 ± 0.2 μM | U. bilgramii | Umbilicaria sp. | Luan et al., 2020 [52] |
| Polyketides | lecanicillone A (257) | fresh weight and root elongation of Arabidopsis thaliana | 32.04 μM | Phaeosphaeria sp. | black lichen | Zhai et al., 2023 [64] |
| Polyketides | lecanicillolide (258) | fresh weight and root elongation of Arabidopsis thaliana | 26.78 μM | Phaeosphaeria sp. | black lichen | Zhai et al., 2023 [64] |
| Terpenoids | ophiokorrin (298) | Root elongation of Arabidopsis thaliana | 18.06 µg/mL | O. korrae | Physciaceae physcia | Li et al., 2019 [30] |
| Steroids | nodulisporiviridin G (377) | Aβ42 aggregation inhibitory activity | 1.2 μM | Nodulisporium sp. | Everniastrum sp. | Zhao et al., 2015 [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ran, Q.; Li, H.; Lou, H. Endolichenic Fungi: A Promising Medicinal Microbial Resource to Discover Bioactive Natural Molecules—An Update. J. Fungi 2024, 10, 99. https://doi.org/10.3390/jof10020099
Zhang W, Ran Q, Li H, Lou H. Endolichenic Fungi: A Promising Medicinal Microbial Resource to Discover Bioactive Natural Molecules—An Update. Journal of Fungi. 2024; 10(2):99. https://doi.org/10.3390/jof10020099
Chicago/Turabian StyleZhang, Wenge, Qian Ran, Hehe Li, and Hongxiang Lou. 2024. "Endolichenic Fungi: A Promising Medicinal Microbial Resource to Discover Bioactive Natural Molecules—An Update" Journal of Fungi 10, no. 2: 99. https://doi.org/10.3390/jof10020099
APA StyleZhang, W., Ran, Q., Li, H., & Lou, H. (2024). Endolichenic Fungi: A Promising Medicinal Microbial Resource to Discover Bioactive Natural Molecules—An Update. Journal of Fungi, 10(2), 99. https://doi.org/10.3390/jof10020099