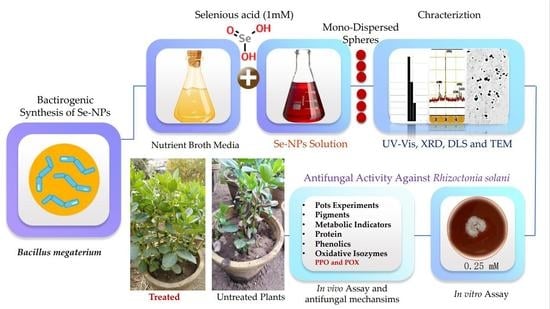
Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosynthesis of Se-NPs
2.2. Characterization of Se-NPs
2.3. Control of Rhizoctonia solani by Se-NPs
2.3.1. Source of Pathogen and Culture Conditions
2.3.2. In Vitro Assessment of Antifungal Activity and Growth Inhibition
- Well diffusion method
- Radial growth method
2.3.3. In Vivo Assessment Efficacy of Se-NPs on Vicia faba
2.3.4. Disease Symptoms and Disease Index
2.4. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Se-NPs
3.2. In Vitro Control of R. solani
3.2.1. Antifungal Activity of Se-NPs and Minimum Inhibition Concentration
3.2.2. Effect of Se-NPs on Linear Growth of R. solani and Minimum Fungicidal Concentration
3.3. In Vivo Control of R. solani
3.3.1. Efficacy of Se-NPs on Rhizoctonia Root Rot Disease of Vicia faba under Pot Conditions
3.3.2. Growth and Yield Responses of Vicia faba by Se-NPs under Pot Conditions
3.3.3. Effect of Se-NPs on Photosynthetic Pigments of Vicia faba under Pot Conditions
3.3.4. Effect of Se-NPs on the Metabolic Indicators of (Vicia faba L.)
Effect of Se-NPs on Phenol Contents of Vicia faba under Pot Conditions
Effect of Se-NPs on a Total Soluble Protein of Vicia faba under Pot Conditions
Effect of Se-NPs on Oxidative Enzymes of Vicia faba under Pot Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015, 74, 62–64. [Google Scholar] [CrossRef]
- Khalil, A.M.A.; Hashem, A.H.; Abdelaziz, A.M. Occurrence of toxigenic Penicillium polonicum in retail green table olives from the Saudi Arabia market. Biocatal. Agric. Biotechnol. 2019, 21, 101314. [Google Scholar] [CrossRef]
- Bramhanwade, K.; Shende, S.; Bonde, S.; Gade, A.; Rai, M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016, 14, 229–235. [Google Scholar] [CrossRef]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boghdady, M.S.; Desoky, E.; Azoz, S.N.; Abdelaziz, D.M. Effect of selenium on growth, physiological aspects and productivity of faba bean (Vicia faba L.). Egypt. J. Agron. 2017, 39, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Gupta, Y. Nutritive value of food legumes. In Chemistry and Biochemistry of Legumes; Arora, S.K., Ed.; Edward Arnold: London, UK, 1983. [Google Scholar]
- Rose, T.J.; Rose, M.T.; Pariasca Tanaka, J.; Heuer, S.; Wissuwa, M. The frustration with utilization: Why have improvements in internal phosphorus utilization efficiency in crops remained so elusive? Front. Plant Sci. 2011, 2, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazen, M.; El-Batanony, N.H.; Abd El-Monium, M.; Massoud, O. Cultural filtrate of Rhizobium spp. and arbuscular mycorrhiza are potential biological control agents against root rot fungal diseases of faba bean. Glob. J. Biotechnol. Biochem. 2008, 3, 32–41. [Google Scholar]
- Elhendawy, S.; Shaban, W.; Sakagami, J.-I. Does treating faba bean seeds with chemical inducers simultaneously increase chocolate spot disease resistance and yield under field conditions? Turk. J. Agric. For. 2010, 34, 475–485. [Google Scholar]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crop. Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Nebiyu, A.; Diels, J.; Boeckx, P. Phosphorus use efficiency of improved faba bean (Vicia faba) varieties in low-input agro-ecosystems. J. Plant Nutr. Soil Sci. 2016, 179, 347–354. [Google Scholar] [CrossRef]
- Rose, T.J.; Julia, C.C.; Shepherd, M.; Rose, M.T.; Van Zwieten, L. Faba bean is less susceptible to fertiliser N impacts on biological N2 fixation than chickpea in monoculture and intercropping systems. Biol. Fertil. Soils 2016, 52, 271–276. [Google Scholar] [CrossRef]
- Erper, I.; Ozkoc, I.; Karaca, G.H. Identification and pathogenicity of Rhizoctonia species isolated from bean and soybean plants in Samsun, Turkey. Arch. Phytopathol. Plant Prot. 2011, 44, 78–84. [Google Scholar] [CrossRef]
- Ghosh, S.; Kanwar, P.; Jha, G. Alterations in rice chloroplast integrity, photosynthesis and metabolome associated with pathogenesis of Rhizoctonia solani. Sci. Rep. 2017, 7, 41610. [Google Scholar] [CrossRef]
- Latz, E.; Eisenhauer, N.; Rall, B.C.; Scheu, S.; Jousset, A. Unravelling linkages between plant community composition and the pathogen-suppressive potential of soils. Sci. Rep. 2016, 6, 23584. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.; Khalil, A.; El-Sheikh, H.; Abdel-Rhaman, E.; Hashem, A. Biodegradation and detoxification of bisphenol-A by filamentous fungi screened from nature. J. Adv. Biol. Biotechnol. 2015, 123–132. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 2021, 1–11. [Google Scholar]
- Hashem, A.H.; Suleiman, W.B.; Abu-elreesh, G.; Shehabeldine, A.M.; Khalil, A.M.A. Sustainable lipid production from oleaginous fungus Syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresour. Technol. Rep. 2020, 12, 100569. [Google Scholar] [CrossRef]
- Hashem, A.H.; Suleiman, W.B.; Abu-Elrish, G.M.; El-Sheikh, H.H. Consolidated bioprocessing of sugarcane bagasse to microbial oil by newly isolated Oleaginous Fungus: Mortierella wolfii. Arab. J. Sci. Eng. 2020, 46, 199–211. [Google Scholar] [CrossRef]
- Hashem, A.H.; Hasanin, M.S.; Khalil, A.M.A.; Suleiman, W.B. Eco-green conversion of watermelon peels to single cell oils using a unique oleaginous fungus: Lichtheimia corymbifera AH13. Waste Biomass Valorization 2019, 11, 5721–5732. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H. Eco-friendly, economic fungal universal medium from watermelon peel waste. J. Microbiol. Methods 2020, 168, 105802. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Dong, H.; Xiong, R.; Liang, Y.; Tang, G.; Yang, J.; Tang, J.; Niu, J.; Gao, Y.; Zhou, Z.; Cao, Y. Development of glycine-copper (ii) hydroxide nanoparticles with improved biosafety for sustainable plant disease management. RSC Adv. 2020, 10, 21222–21227. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinsky, A.A.; Gusev, A.A.; Zakharova, O.V.; Klimov, A.I.; Yapryntsev, A.D.; Zherebin, P.M.; Shapoval, O.A.; Lisichkin, G.V. Synthesis of positively charged hybrid PHMB-stabilized silver nanoparticles: The search for a new type of active substances used in plant protection products. Mater. Res. Express 2017, 4, 075018. [Google Scholar] [CrossRef]
- Kaur, P. Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: A review. Biotechnol. Res. Innov. 2018, 2, 63–73. [Google Scholar]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- El-Batal, A.; Haroun, B.M.; Farrag, A.A.; Baraka, A.; El-Sayyad, G.S. Synthesis of silver nanoparticles and incorporation with certain antibiotic using gamma irradiation. J. Pharm. Res. Int. 2014, 1341–1363. [Google Scholar] [CrossRef]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice leaf extract synthesized silver nanoparticles: An in vitro fungicidal evaluation against Rhizoctonia solani, the causative agent of sheath blight disease in rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, H.; Al-Harbi, N.; Karuppiah, P.; Rajaram, S. Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515. [Google Scholar]
- Abdelkareem, M.; Zohri, A.A. Inhibition of three toxigenic fungal strains and their toxins production using selenium nanoparticles. Czech Mycol. 2017, 69, 193–204. [Google Scholar] [CrossRef]
- Shirsat, S.; Kadam, A.; Naushad, M.; Mane, R.S. Selenium nanostructures: Microbial synthesis and applications. RSC Adv. 2015, 5, 92799–92811. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Niu, S.; He, Y.; Wang, G.; Pei, X.; Tao, W.; Wang, M. Preparation, characteristics and feeble induced-apoptosis performance of non-dialysis requiring selenium nanoparticles@ chitosan. Mater. Des. 2019, 182, 108024. [Google Scholar] [CrossRef]
- Alnassar, H.S.; Helal, M.H.; Askar, A.A.; Masoud, D.M.; Abdallah, A.E. Pyridine azo disperse dye derivatives and their selenium nanoparticles (SeNPs): Synthesis, fastness properties, and antimicrobial evaluations. Int. J. Nanomed. 2019, 14, 7903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, M.H.; Refaat, B.M.; Askar, A.A.Z. Marine Streptomyces cyaneus strain Alex-SK121 mediated eco-friendly synthesis of silver nanoparticles using gamma radiation. J. Pharm. Res. Int. 2014, 4, 2525–2547. [Google Scholar]
- El-Batal, A.; Gamal, M.; Ibrahim, M. Biosynthesis of silver nanoparticles using Streptomyces atroverins, strain askar-sh50 and their antimicrobial activity against foodborne pathogens and Mycobacterium tuberculosis. Al Azhar Bull. Sci. March 2017, 9, 87–106. [Google Scholar]
- Tugarova, A.V.; Vetchinkina, E.P.; Loshchinina, E.A.; Burov, A.M.; Nikitina, V.E.; Kamnev, A.A. Reduction of selenite by Azospirillum brasilense with the formation of selenium nanoparticles. Microb. Ecol. 2014, 68, 495–503. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, G.M.; El-Batal, A.; El-Sherbiny, I. Antibacterial potential with molecular docking study against multi-drug resistant bacteria and Mycobacterium tuberculosis of streptomycin produced by Streptomyces atroverins, strain Askar-SH50. J. Chem. Pharm. Res 2017, 9, 189–208. [Google Scholar]
- Ghorab, M.M.; Alqahtani, A.S.; Soliman, A.M.; Askar, A.A. Novel N-(Substituted) Thioacetamide Quinazolinone Benzenesulfonamides as Antimicrobial Agents. Int. J. Nanomed. 2020, 15, 3161. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Hasanin, M.S. Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustain. Chem. Pharm. 2020, 18, 100333. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H.; Abd El-Sayed, E.S.; El-Saied, H. Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: Efficiency and characteristics. Cellulose 2020, 27, 4443–4453. [Google Scholar] [CrossRef]
- Suleiman, W.; El-Sheikh, H.; Abu-Elreesh, G.; Hashem, A. Recruitment of Cunninghamella echinulata as an Egyptian isolate to produce unsaturated fatty acids. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 764–774. [Google Scholar]
- Suleiman, W.; El-Skeikh, H.; Abu-Elreesh, G.; Hashem, A. Isolation and screening of promising oleaginous Rhizopus sp and designing of Taguchi method for increasing lipid production. J. Innov. Pharm. Biol. Sci. 2018, 5, 8–15. [Google Scholar]
- Khalil, A.M.A.; Abdelaziz, A.M.; Khaleil, M.M.; Hashem, A.H. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 2020, 72, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.A.; Hashem, A.H. Morphological changes of conidiogenesis in two Aspergillus species. J Pure Appl Microbiol 2018, 12, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Shubharani, R.; Mahesh, M.; Yogananda Murthy, V. Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of Bee Propolis. J. Nanomed. Nanotechnol. 2019, 10, 2. [Google Scholar]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.-I. Mycogenic selenium nanoparticles as potential new generation broad-spectrum antifungal molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Büttner, G.; Pfähler, B.; Märländer, B. Greenhouse and field techniques for testing sugar beet for resistance to Rhizoctonia root and crown rot. Plant Breed. 2004, 123, 158–166. [Google Scholar] [CrossRef]
- Mousa, A.M.; El-Sayed, S.A. Effect of intercropping and phosphorus fertilizer treatments on incidence of Rhizoctonia root-rot disease of faba bean. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 850–863. [Google Scholar] [CrossRef]
- Grünwald, N.; Coffman, V.; Kraft, J. Sources of partial resistance to Fusarium root rot in the Pisum core collection. Plant Dis. 2003, 87, 1197–1200. [Google Scholar] [CrossRef] [Green Version]
- Alhaithloul, H.A.S.; Attia, M.S.; Abdein, M.A. Dramatic biochemical and anatomical changes in eggplant due to infection with Alternaria solani causing early blight disease. Int. J. Bot. Stud. 2019, 4, 55–60. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University USA: Ames, IA, USA, 1980; pp. 80–86. [Google Scholar]
- Cittrarasu, V.; Kaliannan, D.; Dharman, K.; Maluventhen, V.; Easwaran, M.; Liu, W.C.; Balasubramanian, B.; Arumugam, M. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Abu-Elghait, M.; Hasanin, M.; Hashem, A.H.; Salem, S.S. Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. Int. J. Biol. Macromol. 2021, 175, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, M.M.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J. Clust. Sci. 2020, 32, 351–361. [Google Scholar] [CrossRef]
- Zare, B.; Babaie, S.; Setayesh, N.; Shahverdi, A.R. Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomed. J. 2013, 1, 13–19. [Google Scholar]
- Ramamurthy, C.; Sampath, K.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, S.; Cameotra, S.S. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Factories 2010, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oremland, R.S.; Herbel, M.J.; Blum, J.S.; Langley, S.; Beveridge, T.J.; Ajayan, P.M.; Sutto, T.; Ellis, A.V.; Curran, S. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl. Environ. Microbiol. 2004, 70, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Min, J.S.; Kim, K.S.; Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, S.B.; Jung, M.; Lee, Y.S. Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant Pathol. J. 2009, 25, 376–380. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.; Patiño-Portela, M.; Mosquera-Sánchez, L.; Guerrero-Vargas, J.; Rodríguez-Páez, J. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef] [Green Version]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, N.; Ismail, A.M. The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar] [CrossRef]
- Ismail, A.-W.A.; Sidkey, N.M.; Arafa, R.A.; Fathy, R.M.; El-Batal, A.I. Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against Alternaria solani caused early blight disease on potato. Biotechnol. J. Int. 2016, 1–11. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Freeman, J.L.; Lindblom, S.D.; Quinn, C.F.; Fakra, S.; Marcus, M.A.; Pilon-Smits, E.A. Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytol. 2007, 175, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Monaim, M.F. Improvement of biocontrol of damping-off and root rot/wilt of faba bean by salicylic acid and hydrogen peroxide. Mycobiology 2013, 41, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akladious, S.A.; Gomaa, E.Z.; El-Mahdy, O.M. Efficiency of bacterial biosurfactant for biocontrol of Rhizoctonia solani (AG-4) causing root rot in faba bean (Vicia faba) plants. Eur. J. Plant Pathol. 2019, 153, 15–35. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Osvald, J. Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol. Biochem. 2005, 43, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, M.; Turakainen, M.; Hartikainen, H. Selenium effects on oxidative stress in potato. Plant Sci. 2003, 165, 311–319. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [Green Version]
- Pezzarossa, B.; Remorini, D.; Gentile, M.L.; Massai, R. Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J. Sci. Food Agric. 2012, 92, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Dehgahi, R.; Subramaniam, S.; Zakaria, L.; Joniyas, A.; Firouzjahi, F.B.; Haghnama, K.; Razinataj, M. Review of research on fungal pathogen attack and plant defense mechanism against pathogen. Int. J. Sci. Res. Agric. Sci. 2015, 2, 197–208. [Google Scholar] [CrossRef]
- Saha, P.; Raychaudhuri, S.S.; Chakraborty, A.; Sudarshan, M. PIXE analysis of trace elements in relation to chlorophyll concentration in Plantago ovata Forsk. Appl. Radiat. Isot. 2010, 68, 444–449. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.; Gao, D.; Liu, G.; Bai, H.; Li, A.; Peng, L.; Ren, Z. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Večeřová, K.; Večeřa, Z.; Dočekal, B.; Oravec, M.; Pompeiano, A.; Tříska, J.; Urban, O. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. Pollut. 2016, 218, 207–218. [Google Scholar] [CrossRef]
- Boyer, R.F.; Clark, H.M.; LaRoche, A.P. Reduction and release of ferritin iron by plant phenolics. J. Inorg. Biochem. 1988, 32, 171–181. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M.; Hamzah, M.H.; Shukor, N.I.A. The causal agent of anthracnose in papaya fruit and control by three different Malaysian stingless bee honeys, and the chemical profile. Sci. Hortic. 2019, 257, 108590. [Google Scholar] [CrossRef]
- Lewis, N.G.; Yamamoto, E. Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 2001, 34, 135–148. [Google Scholar] [CrossRef]
- Mellersh, D.G.; Foulds, I.V.; Higgins, V.J.; Heath, M.C. H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J. 2002, 29, 257–268. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Jones, P. Phytoplasmas: Genomes, Plant Hosts And; Cabi: New York, NY, USA, 2009. [Google Scholar]
- Siddique, Z.; Akhtar, K.P.; Hameed, A.; Sarwar, N.; Imran-Ul-Haq; Khan, S.A. Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. J. Plant Interact. 2014, 9, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Hajiboland, R. Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants. Acta Agric. Slov. 2012, 99, 13. [Google Scholar] [CrossRef]
- Mahfouze, H.A.; El-Dougdoug, N.K.; Mahfouze, S.A. Virucidal activity of silver nanoparticles against Banana bunchy top virus (BBTV) in banana plants. Bull. Natl. Res. Cent. 2020, 44, 199. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.-A.A.; Darwesh, O.M.; Mekki, B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef] [PubMed]








| Treatment Number | Treatment |
|---|---|
| 1 (Control healthy) | The sterilized Vicia faba seeds submerged in distilled water for three hours and sowing in sterilized soil. |
| 2 (Control infected) | Sowing the sterilized Vicia faba seeds in distilled water for three hours and sowing in inoculated soil with R. solani. |
| 3 (Healthy + Nano soaking) | Soaking the sterilized Vicia faba seeds in Se-NPs (0.0625 mM) for three hours and sowing in sterilized soil. |
| 4 (Infected + soaking) | Soaking the sterilized Vicia faba seeds in Se-NPs (0.0625 mM) for three hours and sowing in inoculated soil with R. solani. |
| 5 (Healthy + soaking and spraying with Nano) | Soaking the sterilized Vicia faba seeds in Se-NPs (0.0625 mM) for three hours and sowing in sterilized soil, then spraying 15 mL of Se-NPs after emergence. |
| 6 (Infected + soaking and spraying with Nano) | Soaking the sterilized Vicia faba seeds in Se-NPs (0.0625 mM) for three hours and sowing in inoculated soil with R. solani, then spraying 15 mL of Se-NPs after emergence. |
| 7 (Healthy + spraying Nano) | Sowing the sterilized Vicia faba seeds in distilled water for three hours and sowing in sterilized soil, then spraying 15 mL of Se-NPs (0.0625 mM) after emergence. |
| 8 (Infected + spraying Nano) | Sowing the sterilized Vicia faba seeds in distilled water for three hours and sowing in inoculated soil with R. solani, then spraying 15 mL of Se-NPs (0.0625 mM) after emergence. |
| Treatment | Pre-Emergence Damping of % | Post-Emergence Damping of % | Survival Plant % | Disease Index % | Protection % | |
|---|---|---|---|---|---|---|
| Healthy | Control | 0 | 0 | 100 | 0 | - |
| Nano soaking | 0 | 0 | 100 | 0 | - | |
| Nano spraying | 0 | 0 | 100 | 0 | - | |
| Nano (soaking + spraying) | 0 | 0 | 100 | 0 | - | |
| Infected | Control | 50 | 8.33 | 41.67 | 88 | 0 |
| Nano soaking | 16.66 | 16.66 | 66.67 | 36 | 59.2 | |
| Nano spraying | 50 | 0 | 50 | 32 | 63.63 | |
| Nano (soaking + spraying) | 16.667 | 0 | 83.33 | 20 | 77.27 | |
| Treatments | Plant Height (cm) | Root Length (cm) | Number of Leaves | Shoot F. wt. (g) | Shoot D. wt. (g) | Root F. wt. (g) | Root F. wt. (g) |
|---|---|---|---|---|---|---|---|
| T1: Control (H.) | 32 ± 1.50 b | 11.33 ± 0.85 cd | 16.33 ± 0.57 bc | 10.48 ± 0.72 bc | 3.71 ± 0.24 b | 1.25 ± 0.04 d | 0.37 ± 0.00 bc |
| T2: Control (Inf.) | 19.36 ± 0.70 e | 9.73 ± 0.75 d | 11.66 ± 0.57 d | 7.38 ± 0.33 e | 1.31 ± 0.28 d | 0.82 ± 0.06 g | 0.19 ± 0.02 d |
| T3: Soaking Nano (H.) | 35.83 ± 1.89 b | 13.56 ± 0.51 ab | 18.33 ± 1.52 b | 13.56 ± 0.40 a | 4.6 ± 0.35 a | 1.64 ± 0.06 b | 0.52 ± 0.04 a |
| T4: Soaking Nano (Inf.) | 20.5 ± 1.80 de | 10.7 ± 0.75 cd | 15.33 ± 1.15 c | 10.25 ± 0.67 bc | 2.48 ± 0.14 c | 1.07 ± 0.06 ef | 0.22 ± 0.00 d |
| T5: Soaking + Spray Nano (H.) | 42.66 ± 2.25 a | 15.66 ± 1.10 a | 22.33 ± 1.52 a | 14.76 ± 0.92 a | 4.59 ± 0.22 a | 1.87± 0.03 a | 0.57 ± 0.04 a |
| T6: Soaking + Spray Nano (Inf.) | 24.5 ± 0.50 cd | 10.84 ± 0.74 cd | 11.66 ± 0.57 d | 8.37± 0.10 de | 2.67 ± 0.30 c | 0.93 ± 0.06 fg | 0.31 ± 0.00 c |
| T7: Spray Nano (H.) | 34.5 ± 1.32 b | 12.06 ± 0.62 bc | 16.33 ± 0.57 bc | 11.16 ± 0.35 b | 3.92 ± 0.13 ab | 1.45 ± 0.09 c | 0.44 ± 0.00 b |
| T8: Spray Nano (Inf.) | 26.66 ± 1.52 c | 10.6 ± 0.65 cd | 13.66 ± 0.57 cd | 9.46 ± 0.47 cd | 2.65 ± 0.16 c | 1.13 ± 0.01 de | 0.35 ± 0.00 c |
| L.S.D at 0.05 | 2.667 | 1.329 | 1.694 | 0.963 | 0.422 | 0.099 | 0.041 |
| Treatments | No. of Pods/Plant | No. of Seeds/Plant | wt. of 100 Seeds(g) | Protein Yield mg/g (g) |
|---|---|---|---|---|
| T1: Control (H.) | 20.33 ± 0.57 a | 51 ± 1.0 bc | 101.33 ± 0.57 c | 116.94 ± 0.09 c |
| T2: Control (Inf.) | 17.66 ± 0.57 b | 47 ± 2.0 c | 99 ± 1.0 d | 96.1 ± 0.28 f |
| T3: Soaking Nano (H.) | 21.33 ± 0.57 a | 54.66 ± 1.52 ab | 105 ± 1.0 b | 120.6 ± 0.46 b |
| T4: Soaking Nano (Inf.) | 18.33 ± 0.57 b | 49 ± 1.73 c | 101.66 ± 0.57 c | 99.02 ± 0.14 e |
| T5: Soaking + Spray Nano (H.) | 21.66 ± 0.57 a | 57.66 ± 1.15 a | 107.03 ± 0.45 a | 123.21 ± 0.38 a |
| T6: Soaking + Spray Nano (Inf.) | 17.33 ± 0.57 b | 49 ± 1.73 c | 101.16 ± 0.28 c | 101.28 ± 0.51 d |
| T7: Spray Nano (H.) | 20.33 ± 0.57 a | 54.33 ± 1.15 ab | 103.5 ± 0.05 b | 118.17 ± 0.32 c |
| T8: Spray Nano (Inf.) | 18 ± 1.0 b | 51 ± 1.0 bc | 101.30 ± 0.02 c | 100.21 ± 1.05 de |
| L.S.D at 0.05 | 1.125 | 2.523 | 1.095 | 0.856 |
| Treatments | Protein Shoot mg/g d. wt. (g) | Protein Root mg/g d. wt. (g) |
|---|---|---|
| T1: Control (H.) | 20.22 ± 0.15 c | 18.27 ± 0.17 c |
| T2: Control (Inf.) | 16.8 ± 0.24 f | 16.63 ± 0.35 f |
| T3: Soaking Nano (H.) | 21.83 ± 0.06 b | 19.14 ± 0.17 b |
| T4: Soaking Nano (Inf.) | 18.06 ± 0.48 e | 17.39 ± 0.07 e |
| T5: Soaking + Spray Nano (H.) | 22.93 ± 0.05 a | 21.03 ± 0.07 a |
| T6: Soaking+ Spray Nano (Inf.) | 19.09 ± 0.08 d | 17.59 ± 0.07 de |
| T7: Spray Nano (H.) | 21.62 ± 0.04 b | 20.9 ± 0.09 a |
| T8: Spray Nano (Inf.) | 18.08 ± 0.31 e | 18 ± 0.17 cd |
| LSD at 0.05 | 0.412 | 0.283 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. https://doi.org/10.3390/jof7030195
Hashem AH, Abdelaziz AM, Askar AA, Fouda HM, Khalil AMA, Abd-Elsalam KA, Khaleil MM. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. Journal of Fungi. 2021; 7(3):195. https://doi.org/10.3390/jof7030195
Chicago/Turabian StyleHashem, Amr H., Amer M. Abdelaziz, Ahmed A. Askar, Hossam M. Fouda, Ahmed M. A. Khalil, Kamel A. Abd-Elsalam, and Mona M. Khaleil. 2021. "Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants" Journal of Fungi 7, no. 3: 195. https://doi.org/10.3390/jof7030195
APA StyleHashem, A. H., Abdelaziz, A. M., Askar, A. A., Fouda, H. M., Khalil, A. M. A., Abd-Elsalam, K. A., & Khaleil, M. M. (2021). Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. Journal of Fungi, 7(3), 195. https://doi.org/10.3390/jof7030195