Nanocarriers of Miconazole or Fluconazole: Effects on Three-Species Candida Biofilms and Cytotoxic Effects In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Assembly and Characterization of the Nanocarriers
2.2. Effects of the Nanocarriers on Three-Species Candida Biofilms
2.2.1. Microorganisms and Culture Conditions
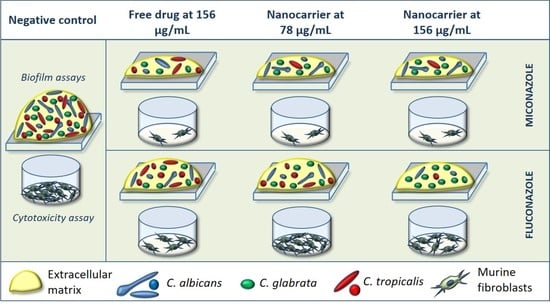
2.2.2. Biofilm Formation and Treatment with the Nanocarriers
2.2.3. Biofilm Quantification
2.3. Cytotoxic Effect of the Nanocarriers on Murine Fibroblasts
2.4. Statistical Analysis
3. Results
3.1. Effects of the Nanocarriers on Three-Species Candida Biofilms
3.2. Cytotoxic Effect of the Nanocarriers on Murine Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcos-Arias, C.; Vicente, J.L.; Sahand, I.H.; Eguia, A.; De-Juan, A.; Madariaga, L.; Aguirre, J.M.; Eraso, E.; Quindós, G. Isolation of Candida dubliniensis in denture stomatitis. Arch. Oral Biol. 2009, 54, 127–131. [Google Scholar] [CrossRef]
- Tay, L.Y.; Jorge, J.H.; Herrera, D.R.; Campanha, N.H.; Gomes, B.P.; Dos Santos, F.A. Evaluation of different treatment meth-ods against denture stomatitis: A randomized clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 72–77. [Google Scholar] [CrossRef]
- Barbosa, A.H.; Damasceno, J.L.; Casemiro, L.A.; Martins, C.H.G.; Pires, R.H.; Candido, R.C. Susceptibility to oral antiseptics and virulence factors ex vivo associated with Candida spp. isolated from dental prostheses. J. Prosthodont. 2019, 28, 398–408. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chu, W.-L.; Lin, C.-C.; Tsai, S.-H.; Chang, T.-P.; Lo, H.-J. An emerging issue of mixed yeast cultures. J. Microbiol. Immunol. Infect. 2014, 47, 339–344. [Google Scholar] [CrossRef]
- Quindos, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef]
- Sawant, B.; Khan, T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical im-pact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tverdek, F.P.; Kofteridis, D.; Kontoyiannis, D.P. Antifungal agents and liver toxicity: A complex interaction. Expert Rev. Anti-Infect. Ther. 2016, 14, 765–776. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Piktel, E.; Bucki, R. Development of antifungal therapies using nanomaterials. Nanomedicine 2017, 12, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.D.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- De Lima, T.M.; Arias, L.S.; Afanaci, L.F.; Ferraresse, R.F.; de S Neto, F.N.; de Lima, B.H.; Straioto, F.G.; De Camargo, E.R.; Pessan, J.P.; Monteiro, D.R. Assembly and antifungal effect of a new fluconazole-carrier nanosystem. Future Microbiol. 2020, 15, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; de Souza Neto, F.N.; de Lima, B.H.R.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.B.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080. [Google Scholar] [CrossRef]
- Lamfon, H.; Porter, S.R.; McCullough, M.; Pratten, J. Formation of Candida albicans biofilms on non-shedding oral surfaces. Eur. J. Oral Sci. 2003, 111, 465–471. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Delbem, A.C.B.; Fernandes, R.A.; Barbosa, D.B.; Monteiro, D.R. Activity of tyrosol against single and mixed-species oral biofilms. J. Appl. Microbiol. 2016, 120, 1240–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamiya, A.S.; Monteiro, D.; Bernabé, D.G.; Gorup, L.F.; Camargo, E.R.; Gomes-Filho, J.E.; Oliveira, S.; Barbosa, D.B. In Vitro and In Vivo Toxicity Evaluation of Colloidal Silver Nanoparticles Used in Endodontic Treatments. J. Endod. 2016, 42, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Abbas, H.S.; Krishnan, A. Magnetic Nanosystems as a Therapeutic Tool to Combat Pathogenic Fungi. Adv. Pharm. Bull. 2020, 10, 512–523. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [Green Version]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination Therapy to Treat Fungal Biofilm-Based Infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef]
- Chatelon, J.; Cortegiani, A.; Hammad, E.; Cassir, N.; Leone, M. Choosing the Right Antifungal Agent in ICU Patients. Adv. Ther. 2019, 36, 3308–3320. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.F.; Rodrigues, M.E.; Silva, S.C.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.; Kastora, S.L.; Gomes-Gonçalves, A.; Azevedo, N.; Rodrigues, C.F.; Silva, S.; Demuyser, L.; Van Dijck, P.; Casal, M.; Brown, A.J.P.; et al. Transcriptional responses of Candida glabrata biofilm cells to fluconazole are modulated by the carbon source. NPJ Biofilms Microbiomes 2020, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Araujo, H.C.; da Silva, A.C.G.; Paião, L.I.; Magario, M.K.W.; Frasnelli, S.C.T.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Antimicrobial, antibiofilm and cytotoxic effects of a colloidal nanocarrier composed by chitosan-coated iron oxide nanoparticles loaded with chlorhexidine. J. Dent. 2020, 101, 03453. [Google Scholar] [CrossRef] [PubMed]
- Araujo, H.C.; Arias, L.S.; Caldeirão, A.C.M.; Assumpção, L.C.D.F.; Morceli, M.G.; de Souza Neto, F.N.; de Camargo, E.R.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Novel colloidal nanocarrier of cetylpyridinium chloride: Antifungal activities on Candida species and cytotoxic potential on murine fibroblasts. J. Fungi 2020, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Süloğlu, A.K.; Karacaoğlu, E.; Koçkaya, E.A.; Selmanoğlu, G.; Loğoglu, E. Cytotoxic effects of a novel thialo benzene deriva-tive 2,4-dithiophenoxy-1-iodo-4-bromobenzene (C18H12S2IBr) in L929 cells. Int. J. Toxicol. 2014, 33, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.M.D.S.; Mota, T.C.; Guimarães, A.C.; Bonfim, L.T.; Burbano, R.R.; Bahia, M.D.O. Cytotoxic and Genotoxic Effects of Fluconazole on African Green Monkey Kidney (Vero) Cell Line. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Lam, P.L.; Wong, M.M.; Hung, L.K.; Yung, L.H.; Tang, J.O.; Lam, K.H.; Chung, P.Y.; Wong, W.Y.; Ho, Y.W.; Wong, R.S.M.; et al. Miconazole and terbinafine induced reactive oxygen species accumulation and topical toxicity in human keratinocytes. Drug Chem. Toxicol. 2020, 1–5. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Shiau, M.Y.; Ou, Y.C.; Huang, Y.C.; Chen, C.C.; Cheng, C.L.; Chiu, K.Y.; Wang, S.S.; Tsai, K.J. Miconazole induces apoptosis via the death receptor 5-dependent and mitochondrial-mediated pathways in human bladder cancer cells. Oncol. Rep. 2017, 37, 3606–3616. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldeirão, A.C.M.; Araujo, H.C.; Arias, L.S.; Ramírez Carmona, W.; Miranda, G.P.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Nanocarriers of Miconazole or Fluconazole: Effects on Three-Species Candida Biofilms and Cytotoxic Effects In Vitro. J. Fungi 2021, 7, 500. https://doi.org/10.3390/jof7070500
Caldeirão ACM, Araujo HC, Arias LS, Ramírez Carmona W, Miranda GP, Oliveira SHP, Pessan JP, Monteiro DR. Nanocarriers of Miconazole or Fluconazole: Effects on Three-Species Candida Biofilms and Cytotoxic Effects In Vitro. Journal of Fungi. 2021; 7(7):500. https://doi.org/10.3390/jof7070500
Chicago/Turabian StyleCaldeirão, Anne Caroline Morais, Heitor Ceolin Araujo, Laís Salomão Arias, Wilmer Ramírez Carmona, Gustavo Porangaba Miranda, Sandra Helena Penha Oliveira, Juliano Pelim Pessan, and Douglas Roberto Monteiro. 2021. "Nanocarriers of Miconazole or Fluconazole: Effects on Three-Species Candida Biofilms and Cytotoxic Effects In Vitro" Journal of Fungi 7, no. 7: 500. https://doi.org/10.3390/jof7070500
APA StyleCaldeirão, A. C. M., Araujo, H. C., Arias, L. S., Ramírez Carmona, W., Miranda, G. P., Oliveira, S. H. P., Pessan, J. P., & Monteiro, D. R. (2021). Nanocarriers of Miconazole or Fluconazole: Effects on Three-Species Candida Biofilms and Cytotoxic Effects In Vitro. Journal of Fungi, 7(7), 500. https://doi.org/10.3390/jof7070500