Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste
Abstract
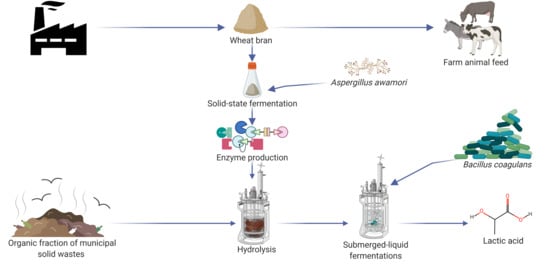
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Microorganisms and Culture Conditions
2.3. Solid State Fermentation, Hydrolyses and Lactic Acid Fermentation
2.3.1. Solid State Fermentation
2.3.2. Hydrolysis Processes
2.3.3. Lactic Acid Fermentation
2.4. Enzymatic Extract
2.4.1. Total Reducing Sugar Test
2.4.2. Enzymes Activity
2.5. pH Measurements
2.6. Analytical Assays
3. Results and Discussion
3.1. Solid State Fermentation, Substrate Optimization and Enzymes Production
3.2. Effect of Fermentation Time on Enzymes Activity
3.3. Hydrolysis Optimization
3.4. Hydrolysate Characterization and Lactic Acid Production
3.5. Mass Balance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khandelwal, H.; Dhar, H.; Thalla, A.K.; Kumar, S. Application of life cycle assessment in municipal solid waste management: A worldwide critical review. J. Clean. Prod. 2019, 209, 630–654. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Latorre-Sanchez, M.; Unger, P.; Schneider, R.; Lozano, C.C.; Venus, J. Assessing the organic fraction of municipal solid wastes for the production of lactic acid. Biochem. Eng. J. 2019, 150, 107251. [Google Scholar] [CrossRef]
- Szabo, K.; Emőke Teleky, B.; Ranga, F.; Simon, E.; Lelia Pop, O.; Babalau-Fuss, V.; Kapsalis, N.; Cristian Vodnar, D. Bioaccessibility of microencapsulated carotenoids, recovered from tomato processing industrial by-products, using in vitro digestion model. LWT 2021, 152, 112285. [Google Scholar] [CrossRef]
- Martau, G.A.; Calinoiu, L.F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- Mmereki, D.; Baldwin, A.; Li, B. A comparative analysis of solid waste management in developed, developing and lesser developed countries. Environ. Technol. Rev. 2016, 5, 120–141. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sanchez, M.; Lozano, C.C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Papa, G.; Pepe Sciarria, T.; Carrara, A.; Scaglia, B.; D’Imporzano, G.; Adani, F. Implementing polyhydroxyalkanoates production to anaerobic digestion of organic fraction of municipal solid waste to diversify products and increase total energy recovery. Bioresour. Technol. 2020, 318, 124270. [Google Scholar] [CrossRef] [PubMed]
- Korkakaki, E.; Mulders, M.; Veeken, A.; Rozendal, R.; van Loosdrecht, M.C.; Kleerebezem, R. PHA production from the organic fraction of municipal solid waste (OFMSW): Overcoming the inhibitory matrix. Water Res. 2016, 96, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Allegue, L.D.; Puyol, D.; Melero, J.A. Novel approach for the treatment of the organic fraction of municipal solid waste: Coupling thermal hydrolysis with anaerobic digestion and photo-fermentation. Sci. Total Environ. 2020, 714, 136845. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Stefanescu, B.E.; Pop, I.D.; Vodnar, D.C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol into 1,3-Propanediol. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Shin, H.S. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrogen Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Sanders, J.; Scott, E.; Weusthuis, R.; Mooibroek, H. Bio-refinery as the bio-inspired process to bulk chemicals. Macromol. Biosci. 2007, 7, 105–117. [Google Scholar] [CrossRef]
- Sakai, K.; Ezaki, Y. Open L-lactic acid fermentation of food refuse using thermophilic Bacillus coagulans and fluorescence in situ hybridization analysis of microflora. J. Biosci. Bioeng. 2006, 101, 457–463. [Google Scholar] [CrossRef]
- Ohkouchi, Y.; Inoue, Y. Impact of chemical components of organic wastes on L(+)-lactic acid production. Bioresour. Technol. 2007, 98, 546–553. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Mitrea, L.; Calinoiu, L.F.; Martau, G.A.; Szabo, K.; Teleky, B.E.; Muresan, V.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef] [Green Version]
- Arte, E.; Huang, X.; Nordlund, E.; Katina, K. Biochemical characterization and technofunctional properties of bioprocessed wheat bran protein isolates. Food Chem. 2019, 289, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Kong, Q.; Chi, C.; Shan, S.; Guan, B. Biotransformation of aflatoxin B1 and aflatoxin G1 in peanut meal by anaerobic solid fermentation of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Food Microbiol. 2015, 211, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.S.; de Almeida, S.S.; Cavalcanti, E.D.C.; Freire, D.M.G.; Moura-Nunes, N.; Monteiro, M.; Perrone, D. Enzymes produced by solid state fermentation of agro-industrial by-products release ferulic acid in bioprocessed whole-wheat breads. Food Res. Int. 2021, 140, 109843. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Oberoi, H.S.; Kaur, S.; Bansal, S.; Brar, S.K. Value-addition of agricultural wastes for augmented cellulase and xylanase production through solid-state tray fermentation employing mixed-culture of fungi. Ind. Crop. Prod. 2011, 34, 1160–1167. [Google Scholar] [CrossRef]
- Rosales, E.; Pazos, M.; Ángeles Sanromán, M. Solid-state fermentation for food applications. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 319–355. [Google Scholar] [CrossRef]
- Camassola, M.; Dillon, A.J. Cellulases and xylanases production by Penicillium echinulatum grown on sugar cane bagasse in solid-state fermentation. Appl. Biochem. Biotechnol. 2010, 162, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Bourgine, J.; Thomsen, M. Closing the loop of cereal waste and residues with sustainable technologies: An overview of enzyme production via fungal solid-state fermentation. Sustain. Prod. Consum. 2021, 27, 845–857. [Google Scholar] [CrossRef]
- Outeirino, D.; Costa-Trigo, I.; Oliveira, R.P.D.; Guerra, N.P.; Dominguez, J.M. A novel approach to the biorefinery of brewery spent grain. Process. Biochem. 2019, 85, 135–142. [Google Scholar] [CrossRef]
- Kornbrust, B.A.; Forman, T.; Matveeva, I. Applications of Enzymes in Breadmaking; Woodhead Publishing: Cambridge, UK, 2012; pp. 470–498. [Google Scholar] [CrossRef]
- Anto, H.; Trivedi, U.B.; Patel, K.C. Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour. Technol. 2006, 97, 1161–1166. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wang, Q.H.; Liu, Y.Y.; Ma, H.Z. On-site production of crude glucoamylase for kitchen waste hydrolysis. Waste Manag. Res. 2010, 28, 539–544. [Google Scholar] [CrossRef]
- Osho, M.B.; Solomon, T. Use of composite agro-substrates for amyloglucosidase synthesis and characterization by Aspergillus niger otf and Aspergillus flavus clor1 using solid state fermentation. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 879–883. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Stepwise optimisation of enzyme production in solid state fermentation of waste bread pieces. Food Bioprod. Process. 2013, 91, 638–646. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Perez-Rivero, C.; Venus, J. Valorisation of solid biowastes: The lactic acid alternative. Process Biochem. 2020, 99, 222–235. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Unger, P.; Schneider, R.; Venus, J. From Upstream to Purification: Production of Lactic Acid from the Organic Fraction of Municipal Solid Waste. Waste Biomass Valorization 2020, 11, 5247–5254. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Cui, Y.; Cheng, Q.; Zhang, Z.; Lu, J.H.; Meng, Q.; Teng, L.; Ren, X. Measurement of filter paper activities of cellulase with microplate-based assay. Saudi. J. Biol. Sci. 2016, 23, S93–S98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef]
- Tiwari, R.; Nain, P.K.S.; Singh, S.; Adak, A.; Saritha, M.; Rana, S.; Sharma, A.; Nain, L. Cold active holocellulase cocktail from Aspergillus niger SH3: Process optimization for production and biomass hydrolysis. J. Taiwan Inst. Chem. Eng. 2015, 56, 57–66. [Google Scholar] [CrossRef]
- Ma, F.Y.; Wang, J.J.; Zeng, Y.L.; Yu, H.B.; Yang, Y.; Zhang, X.Y. Influence of the co-fungal treatment with two white rot fungi on the lignocellulosic degradation and thermogravimetry of corn stover. Process. Biochem. 2011, 46, 1767–1773. [Google Scholar] [CrossRef]
- Taherzadeh-Ghahfarokhi, M.; Panahi, R.; Mokhtarani, B. Optimizing the combination of conventional carbonaceous additives of culture media to produce lignocellulose-degrading enzymes by Trichoderma reesei in solid state fermentation of agricultural residues. Renew. Energy 2019, 131, 946–955. [Google Scholar] [CrossRef]
- Pandey, A.K.; Edgard, G.; Negi, S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew. Energy 2016, 98, 51–56. [Google Scholar] [CrossRef]
- Kaushik, P.; Mishra, A.; Malik, A. Dual application of agricultural residues for xylanase production and dye removal through solid state fermentation. Int. Biodeterior. Biodegrad. 2014, 96, 1–8. [Google Scholar] [CrossRef]
- Thomas, L.; Parameswaran, B.; Pandey, A. Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp for bioethanol production. Renew. Energy 2016, 98, 9–15. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Deswal, D.; Karp, M.; Kuhad, R.C. Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel 2014, 124, 183–189. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Babbar, N.; Dhaliwal, S.S.; Kaur, S.; Vadlani, P.V.; Bhargav, V.K.; Patil, R.T. Enhanced Oil Recovery by Pre-treatment of Mustard Seeds Using Crude Enzyme Extract Obtained from Mixed-Culture Solid-State Fermentation of Kinnow (Citrus reticulata) Waste and Wheat Bran. Food Bioprocess. Technol. 2010, 5, 759–767. [Google Scholar] [CrossRef]
- Singhania, R.R.; Sukumaran, R.K.; Patel, A.K.; Larroche, C.; Pandey, A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzym. Microb. Technol. 2010, 46, 541–549. [Google Scholar] [CrossRef]
- Melikoglu, M. Production of Sustainable Alternatives to Petrochemicals and Fuels Using Waste Bread as a Raw Material; The University of Manchester: Manchester, UK, 2008. [Google Scholar]
- López-Gómez, J.P.; Venus, J. Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy. Fermentation 2021, 7, 76. [Google Scholar] [CrossRef]
- Li, Y.B.; Park, S.Y.; Zhu, J.Y. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sust. Energ. Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Probst, M.; Walde, J.; Pumpel, T.; Wagner, A.O.; Insam, H. A closed loop for municipal organic solid waste by lactic acid fermentation. Bioresour. Technol. 2015, 175, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Jem, K.J.; van der Pol, J.F.; de Vos, S. Microbial lactic acid, its polymer poly(lactic acid), and their industrial applications. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 323–346. [Google Scholar] [CrossRef]
- Klotz, S.; Kaufmann, N.; Kuenz, A.; Prusse, U. Biotechnological production of enantiomerically pure d-lactic acid. Appl. Microbiol. Biotechnol. 2016, 100, 9423–9437. [Google Scholar] [CrossRef]
- Kwak, S.; Jin, Y.S. Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: A review and perspective. Microb. Cell Fact. 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teleky, B.E.; Martau, A.G.; Ranga, F.; Chetan, F.; Vodnar, D.C. Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour. Biomolecules 2020, 10, 778. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Bartolucci, S.; Franzen, C.J.; Contursi, P. Bacillus coagulans MA-13: A promising thermophilic and cellulolytic strain for the production of lactic acid from lignocellulosic hydrolysate. Biotechnol. Biofuels 2017, 10, 210. [Google Scholar] [CrossRef] [Green Version]




| Substrate | Total Reducing Sugar (g/kgfs) | ||||
|---|---|---|---|---|---|
| WBE (g) | OFMSW (g) | 0 h | 24 h | 48 h | 68 h |
| 10 | 90 | 4.06 ± 0.23 | 20.99 ± 0.49 | 24.81 ± 0.33 | 28.50 ± 0.49 |
| 20 | 80 | 5.76 ± 0.26 | 24.72 ± 0.41 | 28.85 ± 0.38 | 34.92 ± 0.43 |
| 30 | 70 | 6.28 ± 0.27 | 26.26 ± 0.35 | 31.96 ± 0.46 | 38.32 ± 0.41 |
| 40 | 60 | 9.44 ± 0.33 | 26.91 ± 0.46 | 33.18 ± 0.34 | 39.53 ± 0.42 |
| 50 | 50 | 9.32 ± 0.30 | 27.55 ± 0.45 | 33.02 ± 0.32 | 34.49 ± 0.47 |
| Glucose | Fructose | Disaccharide | Xylose | Arabinose | Lactic Acid | Acetic Acid | ||
|---|---|---|---|---|---|---|---|---|
| Hydrolysis | Initial | 6.64 ± 0.19 | 9.58 ± 0.91 | 3.99 ± 0.74 | 10.18 ± 0.42 | N.D. | 35.98 ± 0.98 | 5.45 ± 0.11 |
| Final | 19.77 ± 1.56 | 9.62 ± 0.36 | 2.65 ± 0.58 | 9.87 ± 0.13 | 0.62 ± 0.12 | 33.30 ± 0.36 | 5.38 ± 0.51 | |
| LA fermentation | Initial | 18.77 ± 1.35 | 8.85 ± 0.31 | 1.88 ± 0.45 | 9.29 ± 0.45 | 0.54 ± 0.16 | 32.38 ± 0.85 | 5.30 ± 0.25 |
| Final | N.D. | 7.06 ± 0.56 | 1.68 ± 0.61 | 7.29 ± 0.32 | N.D. | 47.97 ± 0.37 | 4.98 ± 0.15 | |
| Glucose | Fructose | Disaccharide | Xylose | Arabinose | Lactic Acid | Acetic Acid | ||
|---|---|---|---|---|---|---|---|---|
| Hydrolysis | Initial | 3.68 ± 0.19 | 10.08 ± 1.41 | 4.73 ± 0.30 | 11.14 ± 0.48 | N.D. | 41.22 ± 1.39 | 6.16 ± 0.18 |
| Final | 29.00 ± 0.65 | 10.05 ± 0.57 | 2.63 ± 0.90 | 10.79 ± 0.20 | 0.51 ± 0.15 | 38.52 ± 0.60 | 6.03 ± 0.03 | |
| LA fermentation | Initial | 25.46 ± 1.17 | 9.15 ± 0.30 | 2.26 ± 0.39 | 9.87 ± 0.45 | 0.50 ± 0.17 | 36.44 ± 0.52 | 5.88 ± 0.09 |
| Final | N.D. | 5.28 ± 0.98 | 1.87 ± 0.75 | 7.46 ± 0.78 | N.D. | 57.76 ± 4.41 | 5.39 ± 0.28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martău, G.-A.; Unger, P.; Schneider, R.; Venus, J.; Vodnar, D.C.; López-Gómez, J.P. Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. J. Fungi 2021, 7, 766. https://doi.org/10.3390/jof7090766
Martău G-A, Unger P, Schneider R, Venus J, Vodnar DC, López-Gómez JP. Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. Journal of Fungi. 2021; 7(9):766. https://doi.org/10.3390/jof7090766
Chicago/Turabian StyleMartău, Gheorghe-Adrian, Peter Unger, Roland Schneider, Joachim Venus, Dan Cristian Vodnar, and José Pablo López-Gómez. 2021. "Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste" Journal of Fungi 7, no. 9: 766. https://doi.org/10.3390/jof7090766
APA StyleMartău, G. -A., Unger, P., Schneider, R., Venus, J., Vodnar, D. C., & López-Gómez, J. P. (2021). Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. Journal of Fungi, 7(9), 766. https://doi.org/10.3390/jof7090766