The Sensitivity of Fungi Colonising Buckwheat Grains to Cold Plasma Is Species Specific
Abstract
:1. Introduction
2. Materials and Methods
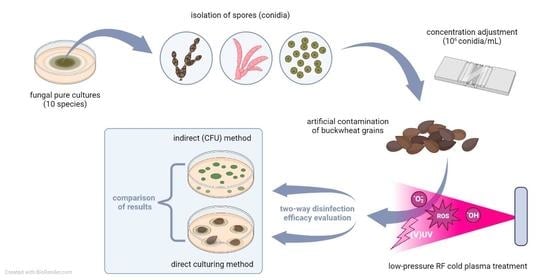
2.1. Source Material
2.2. Uniform Single Fungal Species Inoculation Method for Artificial Contamination of Buckwheat Grains
2.3. Cold Plasma Treatment Conditions
2.4. Evaluation of the Efficiency of the Cold Plasma Decontamination
2.4.1. Direct Cultivation Technique: Contamination Rate
2.4.2. Indirect Cultivation Technique: Colony-Forming Units (CFU)
2.5. Germination Tests
2.6. Statistical Analysis
3. Results
3.1. Cold Plasma Decontamination Efficiency
3.1.1. Direct Cultivation Technique: Contamination Rate
3.1.2. Indirect Evaluation Method: Colony Forming Units (CFU)
3.2. Effect of Cold Plasma on Grain Germination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Gow, N.A.R.; Gurr, S.J. Tackling Emerging Fungal Threats to Animal Health, Food Security and Ecosystem Resilience. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160332. [Google Scholar] [CrossRef] [PubMed]
- Committee, N.A.; Criteria, M. Microbiological Safety Evaluations and Recommendations on Sprouted Seeds. Int. J. Food Microbiol. 1999, 52, 123–153. [Google Scholar] [CrossRef]
- Halloin, J.M. Deterioration Resistance Mechanisms in Seeds. Phytopathology 1983, 73, 335–339. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of Grains and Legumes Infected with Aspergillus Spp. and Penicillum Spp. by Cold Plasma Treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Filtenborg, O.; Frisvad, J.C.; Thrane, U. Moulds in Food Spoilage. Int. J. Food Microbiol. 1996, 33, 85–102. [Google Scholar] [CrossRef]
- Miller, J.D. Fungi and Mycotoxins in Grains: Implication for Stored Product Research. J. Stored Prod. Res. 1995, 31, 1–16. [Google Scholar] [CrossRef]
- Frisvald, J.C.; Samson, R.A. Filamentous Fungi in Foods and Feeds: Ecology, Spoilage, and Mycotoxin Production. In Handbook of Applied Mycology: Volume 3: Foods and Feeds; Arora, D.K., Mukerji, K.G., Marth, E.H., Eds.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold Plasma for Effective Fungal and Mycotoxin Control in Foods: Mechanisms, Inactivation Effects, and Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef]
- Christensen, C.M. Deterioration of Stored Grains by Fungi. Bot. Rev. 1957, 23, 108–134. [Google Scholar] [CrossRef]
- Milevoj, L. Buckwheat Diseases. In Fagopyrum (Buckwheat Newsletter); Kreft, I., Ed.; Biotehniška Fakulteta: Ljubljana, Slovenia, 1989; Volume 9, pp. 31–40. [Google Scholar]
- Kovačec, E.; Likar, M.; Regvar, M. Temporal Changes in Fungal Communities from Buckwheat Seeds and Their Effects on Seed Germination and Seedling Secondary Metabolism. Fungal Biol. 2016, 120, 666–678. [Google Scholar] [CrossRef]
- Singh, P.N.; Sindhu, I.R.; Singhal, G. Fungi Recorded from Seeds and Seedlings of Fagopyrum Esculentum. J. Indian Bot. Soc. 1984, 63, 236–243. [Google Scholar]
- Mills, J.T.; Wallace, H.A.H. Microflora of Buckwheat Seed, Changes in Storage and Effect of Seed Treatments on Seedling Emergence. Can. Plant Dis. Surv. 1971, 51, 154–158. [Google Scholar]
- Mravlje, J.; Regvar, M.; Starič, P.; Mozetič, M.; Vogel-Mikuš, K. Cold Plasma Affects Germination and Fungal Community Structure of Buckwheat Seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L. Some Major Mycotoxins and Their Mycotoxicoses-An Overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Cirlini, M.; Falavigna, C. Mycotoxins from Alternaria: Toxicological Implications, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 8, ISBN 9780444634061. [Google Scholar]
- Konopka, J.; Casadevall, A.; Taylor, J.; Heitman, J.; Cowen, L. One Health: Fungal Pathogens of Humans, Animals, and Plants; American Society of Microbiology: Washington, DC, USA, 2019; Available online: https://asm.org/Reports/One-Health-Fungal-Pathogens-of-Humans,-Animals,-an (accessed on 5 May 2023).
- FAO United Nations. How to Feed the World in 2050 Executive. 2009. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 5 May 2023).
- Mancini, V.; Romanazzi, G. Seed Treatments to Control Seedborne Fungal Pathogens of Vegetable Crops. Pest Manag. Sci. 2014, 70, 860–868. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process. Polym. 2021, 18. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma Agriculture: A Rapidly Emerging Field. Plasma Process. Polym. 2018, 15, 1–5. [Google Scholar] [CrossRef]
- Veerana, M.; Yu, N.; Ketya, W.; Park, G. Application of Non-Thermal Plasma to Fungal Resources. J. Fungi 2022, 8, 102. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Vogel-Mikuš, K. Development of Cold Plasma Technologies for Surface Decontamination of Seed Fungal Pathogens: Present Status and Perspectives. J. Fungi 2021, 7, 650. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Jirešová, J.; Šerá, B.; Julák, J. A Review of Microbial Decontamination of Cereals by Non-Thermal Plasma. Foods 2021, 10, 2927. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Cullen, P.J. Cold Plasma as an Emerging Technique for Mycotoxin-Free Food: Efficacy, Mechanisms, and Trends. Food Rev. Int. 2020, 36, 193–214. [Google Scholar] [CrossRef]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin Decontamination of Food: Cold Atmospheric Pressure Plasma versus “Classic” Decontamination. Toxins 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Conrads, H.; Schmidt, M. Plasma Generation and Plasma Sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric Pressure Plasmas: A Review. Spectrochim. Acta-Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Popović, D.; Mozetič, M.; Vesel, A.; Primc, G.; Zaplotnik, R. Review on Vacuum Ultraviolet Generation in Low-Pressure Plasmas. Plasma Process. Polym. 2021, 18, 2100061. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-Temperature Sterilization Using Gas Plasmas: A Review of the Experiments and an Analysis of the Inactivation Mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-Thermal Plasma Technologies: New Tools for Bio-Decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Starič, P.; Zaplotnik, R.; Mozetič, M.; Vogel-mikuš, K. Decontamination and Germination of Buckwheat Grains upon Treatment with Oxygen Plasma Glow and Afterglow. Plants 2022, 11, 1366. [Google Scholar] [CrossRef]
- Primc, G. Generation of Neutral Chemically Reactive Species in Low-Pressure Plasma. Front. Phys. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Zaplotnik, R.; Vesel, A.; Mozetic, M. A Fiber Optic Catalytic Sensor for Neutral Atom Measurements in Oxygen Plasma. Sensors 2012, 12, 3857–3867. [Google Scholar] [CrossRef] [PubMed]
- Muskett, A.E.; Malone, J.P. The Ulster Method for the Examination of Flax Seed for the Presence of Seed-Borne Parasites. Ann. Appl. Biol. 1941, 28, 8–13. [Google Scholar] [CrossRef]
- Hoppanová, L.; Kryštofová, S. Nonthermal Plasma Effects on Fungi: Applications, Fungal Responses, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 11592. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological Interactions with Cold Plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Lerouge, S.; Wertheimer, M.R.; Yahia, L. Plasma Sterilization: A Review of Parameters, Mechanisms, and Limitations. Plasmas Polym. 2001, 6, 175–188. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Rotolo, C.; Morano, M.; Minafra, A.; Ambrico, M.; Pollastro, S.; Gerin, D.; Faretra, F.; De Miccolis Angelini, R.M. Surface Dielectric Barrier Discharge Plasma: A Suitable Measure against Fungal Plant Pathogens. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma Sterilization. Methods and Mechanisms *. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Philip, N.; Saoudi, B.; Crevier, M.C.; Moisan, M.; Barbeau, J.; Pelletier, J. The Respective Roles of UV Photons and Oxygen Atoms in Plasma Sterilization at Reduced Gas Pressure: The Case of N2-O2 Mixtures. IEEE Trans. Plasma Sci. 2002, 30, 1429–1436. [Google Scholar] [CrossRef]
- Booth, J.P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of Plasma Surface Functionalization of Polymers for Industrial and Biological Applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Bayliss, D.L.; Walsh, J.L.; Iza, F.; Shama, G.; Holah, J.; Kong, M.G. Complex Responses of Microorganisms as a Community to a Flowing Atmospheric Plasma. Plasma Process. Polym. 2012, 9, 597–611. [Google Scholar] [CrossRef]
- Na, Y.H.; Park, G.; Choi, E.H.; Uhm, H.S. Effects of the Physical Parameters of a Microwave Plasma Jet on the Inactivation of Fungal Spores. Thin Solid Films 2013, 547, 125–131. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Shang, H.; Ma, R.; Zhu, Y.; Yang, X.; Ju, S.; Zhao, W.; Sun, H.; Zhuang, J.; et al. Effective Inhibition of Fungal Growth, Deoxynivalenol Biosynthesis and Pathogenicity in Cereal Pathogen Fusarium Spp. by Cold Atmospheric Plasma. Chem. Eng. J. 2022, 437, 135307. [Google Scholar] [CrossRef]
- Szőke, C.; Nagy, Z.; Gierczik, K.; Székely, A.; Spitkól, T.; Zsuboril, Z.T.; Galiba, G.; Marton, C.L.; Kutasi, K. Effect of the Afterglows of Low Pressure Ar/N2-O2 Surface-Wave Microwave Discharges on Barley and Maize Seeds. Plasma Process. Polym. 2018, 15, 1700138. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Xie, H.; Jiang, J.; Li, C.; Li, W.; Li, L.; Liu, X.; Lin, F. Inactivation Effects of Plasma-Activated Water on Fusarium Graminearum. Food Control 2022, 134, 108683. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Intanon, W.; Vichiansan, N.; Leksakul, K.; Boonyawan, D.; Kumla, J.; Suwannarach, N.; Lumyong, S. Inhibition of the Aflatoxin-Producing Fungus Aspergillus Flavus by a Plasma Jet System. J. Food Process. Preserv. 2021, 45, 1–12. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Ni, Y.; Filipič, G.; Cvelbar, U.; Walsh, J.L. Effective Fungal Spore Inactivation with an Environmentally Friendly Approach Based on Atmospheric Pressure Air Plasma. Environ. Sci. Technol. 2019, 53, 1893–1904. [Google Scholar] [CrossRef]
- Sen, Y.; Onal-Ulusoy, B.; Mutlu, M. Aspergillus Decontamination in Hazelnuts: Evaluation of Atmospheric and Low-Pressure Plasma Technology. Innov. Food Sci. Emerg. Technol. 2019, 54, 235–242. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal Plasma Treatment of Aspergillus Spp. Spores on Hazelnuts in an Atmospheric Pressure Fluidized Bed Plasma System: Impact of Process Parameters and Surveillance of the Residual Viability of Spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Makari, M.; Hojjati, M.; Shahbazi, S.; Askari, H. Elimination of Aspergillus Flavus from Pistachio Nuts with Dielectric Barrier Discharge (DBD) Cold Plasma and Its Impacts on Biochemical Indices. J. Food Qual. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of Cold Plasma on Fungal Growth and Aflatoxins Production on Groundnuts. Food Control 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Wiktor, A.; Hrycak, B.; Jasinski, M.; Rybak, K.; Kieliszek, M.; Krasniewska, K.; Witrowa-Rajchert, D. Impact of Atmospheric Pressure Microwave Plasma Treatment on Quality of Selected Spices. Appl. Sci. 2020, 10, 6815. [Google Scholar] [CrossRef]
- Waskow, A.; Betschart, J.; Butscher, D.; Oberbossel, G.; Klöti, D.; Büttner-Mainik, A.; Adamcik, J.; von Rohr, P.R.; Schuppler, M. Characterization of Efficiency and Mechanisms of Cold Atmospheric Pressure Plasma Decontamination of Seeds for Sprout Production. Front. Microbiol. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Nojima, H.; Park, R.E.; Kwon, J.H.; Suh, I.; Jeon, J.; Ha, E.; On, H.K.; Kim, H.R.; Choi, K.; Lee, K.H.; et al. Novel Atmospheric Pressure Plasma Device Releasing Atomic Hydrogen: Reduction of Microbial-Contaminants and OH Radicals in the Air. J. Phys. D Appl. Phys. 2007, 40, 501–509. [Google Scholar] [CrossRef]
- Park, J.C.; Park, B.J.; Han, D.W.; Lee, D.H.; Lee, I.S.; Hyun, S.O.; Chun, M.S.; Chung, K.H.; Aihara, M.; Takatori, K. Fungal Sterilization Using Microwave-Induced Argon Plasma at Atmospheric Pressure. J. Microbiol. Biotechnol. 2004, 14, 188–192. [Google Scholar]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.A.H. Inhibitory Effect of Double Atmospheric Pressure Argon Cold Plasma on Spores and Mycotoxin Production of Aspergillus Niger Contaminating Date Palm Fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S. Do Application of Cold Oxygen Plasma for the Reduction of Cladosporium Cladosporioides and Penicillium Citrinum on the Surface of Dried Filefish (Stephanolepis Cirrhifer) Fillets. Int. J. Food Sci. Technol. 2015, 50, 966–973. [Google Scholar] [CrossRef]
- Julák, J.; Soušková, H.; Scholtz, V.; Kvasničková, E.; Savická, D.; Kříha, V. Comparison of Fungicidal Properties of Non-Thermal Plasma Produced by Corona Discharge and Dielectric Barrier Discharge. Folia Microbiol. (Praha) 2018, 63, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric Pressure Plasma Treatment of Agricultural Seeds of Cucumber (Cucumis Sativus L.) and Pepper (Capsicum Annuum L.) with Effect on Reduction of Diseases and Germination Improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, D.; Song, Y.; Zhou, R.; Niu, J. Inactivation of the Tomato Pathogen Cladosporium Fulvum by an Atmospheric-Pressure Cold Plasma Jet. Plasma Process. Polym. 2014, 11, 1028–1036. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Rocha, L.O.; Oggenfuss, U.; Corrêa, B.; Croll, D.; Rose, L. Complex Evolutionary Origins of Specialized Metabolite Gene Cluster Diversity among the Plant Pathogenic Fungi of the Fusarium Graminearum Species Complex. Genome Biol. Evol. 2019, 11, 3106–3122. [Google Scholar] [CrossRef] [PubMed]
- Fávaro, D.L.C.L.; Melo de, F.L.; Aguilar-Vildoso, C.I.; Araújo, W.L. Polyphasic Analysis of Intraspecific Diversity in Epicoccum Nigrum Warrants Reclassification into Separate Species. PLoS One 2011, 6, e14828. [Google Scholar] [CrossRef]
- Langfelder, K.; Jahn, B.; Gehringer, H.; Schmidt, A.; Wanner, G.; Brakhage, A.A. Identification of a Polyketide Synthase Gene (PksP) of Aspergillus Fumigatus Involved in Conidial Pigment Biosynthesis and Virulence. Med. Microbiol. Immunol. 1998, 187, 79–89. [Google Scholar] [CrossRef]
- Carzaniga, R.; Fiocco, D.; Bowyer, P.; O’Connell, R.J. Localization of Melanin in Conidia of Alternaria Alternata Using Phage Display Antibodies. Mol. Plant-Microbe Interact. 2002, 15, 216–224. [Google Scholar] [CrossRef]
- Ellis, D.H.; Griffiths, D.A. The Location and Analysis of Melanins in the Cell Walls of Some Soil Fungi. Can. J. Microbiol. 1974, 20, 1379–1386. [Google Scholar] [CrossRef]
- Cordero, R.J.B.; Casadevall, A. Functions of Fungal Melanin beyond Virulence Radames. Fungal Biol Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Belozerskaya, T.A.; Gessler, N.N.; Aver’yanov, A.A. Melanin Pigments of Fungi. In Fungal Metabolites; Springer: Cham, Switzerland, 2016; pp. 1–29. ISBN 9783319194561. [Google Scholar]
- Świecimska, M.; Tulik, M.; Šerá, B.; Golińska, P.; Tomeková, J.; Medvecká, V.; Bujdáková, H.; Oszako, T.; Zahoranová, A.; Šerý, M. Non-Thermal Plasma Can Be Used in Disinfection of Scots Pine (Pinus Sylvestris L.) Seeds Infected with Fusarium Oxysporum. Forests 2020, 11, 837. [Google Scholar] [CrossRef]
- Panngom, K.; Lee, S.H.; Park, D.H.; Sim, G.B.; Kim, Y.H.; Uhm, H.S.; Park, G.; Choi, E.H. Non-Thermal Plasma Treatment Diminishes Fungal Viability and up-Regulates Resistance Genes in a Plant Host. PLoS ONE 2014, 9, e99300. [Google Scholar] [CrossRef] [PubMed]
- Homa, K.; Barney, W.P.; Davis, W.P.; Guerrero, D.; Berger, M.J.; Lopez, J.L.; Wyenandt, C.A.; Simon, J.E. Cold Plasma Treatment Strategies for the Control of Fusarium Oxysporum f. Sp. Basilici in Sweet Basil. HortScience 2021, 56, 42–51. [Google Scholar] [CrossRef]
- Go, S.M.; Park, M.R.; Kim, H.S.; Choi, W.S.; Jeong, R.D. Antifungal Effect of Non-Thermal Atmospheric Plasma and Its Application for Control of Postharvest Fusarium Oxysporum Decay of Paprika. Food Control 2019, 98, 245–252. [Google Scholar] [CrossRef]
- Lee, K.; Paek, K.H.; Ju, W.T.; Lee, Y. Sterilization of Bacteria, Yeast, and Bacterial Endospores by Atmospheric-Pressure Cold Plasma Using Helium and Oxygen. J. Microbiol. 2006, 44, 269–275. [Google Scholar]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus Parasiticus from Nut Surface with Low Pressure Cold Plasma (LPCP) Treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Favelukes, G.; Bauer, W.D. Optimization of Surface Sterilization for Legume Seed. Crop Sci. 1990, 30, 708–712. [Google Scholar] [CrossRef]
- Charkowski, A.O.; Sarreal, C.Z.; Mandrell, R.E. Wrinkled Alfalfa Seeds Harbor More Aerobic Bacteria and Are More Difficult to Sanitize than Smooth Seeds. J. Food Prot. 2001, 64, 1292–1298. [Google Scholar] [CrossRef]
- Ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effect of Corona Discharge Plasma Jet Treatment on Decontamination and Sprouting of Rapeseed (Brassica Napus L.) Seeds. Food Control 2017, 71, 376–382. [Google Scholar] [CrossRef]
- Siciliano, I.; Spadaro, D.; Prelle, A.; Vallauri, D.; Cavallero, M.C.; Garibaldi, A.; Gullino, M.L. Use of Cold Atmospheric Plasma to Detoxify Hazelnuts from Aflatoxins. Toxins 2016, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U. Fusarium. In Encyclopedia of Food Microbiology (Second Edition); Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 76–81. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.Y.; Kim, Y.S.; Balaraju, K.; Mok, Y.S.; Yoo, S.J.; Jeon, Y. Enhancement of Seed Germination and Microbial Disinfection on Ginseng by Cold Plasma Treatment. J. Ginseng Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.M.; Münsterkötter, M.; Proctor, R.H.; Brown, D.W.; Sharon, A.; Idan, Y.; Oren-Young, L.; Sieber, C.M.; Novák, O.; Pěnčík, A.; et al. Comparative “Omics” of the Fusarium Fujikuroi Species Complex Highlights Differences in Genetic Potential and Metabolite Synthesis. Genome Biol. Evol. 2016, 8, 3574–3599. [Google Scholar] [CrossRef]
- Jo, Y.K.; Cho, J.; Tsai, T.C.; Staack, D.; Kang, M.H.; Roh, J.H.; Shin, D.B.; Cromwell, W.; Gross, D. A Non-Thermal Plasma Seed Treatment Method for Management of a Seedborne Fungal Pathogen on Rice Seed. Crop Sci. 2014, 54, 796–803. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of Bakanae and Bacterial Seedling Blight Diseases in Nurseries by Irradiating Rice Seeds with Atmospheric Plasma. Plant Pathol. 2017, 66, 67–76. [Google Scholar] [CrossRef]
- Kang, M.H.; Pengkit, A.; Choi, K.; Jeon, S.S.; Choi, H.W.; Shin, D.B.; Choi, E.H.; Uhm, H.S.; Park, G. Differential Inactivation of Fungal Spores in Water and on Seeds by Ozone and Arc Discharge Plasma. PLoS One 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Lee, G.J.; Sim, G.B.; Choi, E.H.; Kwon, Y.W.; Kim, J.Y.; Jang, S.; Kim, S.H. Optical and Structural Properties of Plasma-Treated Cordyceps Bassiana Spores as Studied by Circular Dichroism, Absorption, and Fluorescence Spectroscopy. J. Appl. Phys. 2015, 117, 023303. [Google Scholar] [CrossRef]
- Shapourzadeh, A.; Rahimi-Verki, N.; Atyabi, S.M.; Shams-Ghahfarokhi, M.; Jahanshiri, Z.; Irani, S.; Razzaghi-Abyaneh, M. Inhibitory Effects of Cold Atmospheric Plasma on the Growth, Ergosterol Biosynthesis, and Keratinase Activity in Trichophyton Rubrum. Arch. Biochem. Biophys. 2016, 608, 27–33. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Neutral Reactive Gaseous Species in Reactors Suitable for Plasma Surface Engineering. Surf. Coatings Technol. 2019, 376, 15–20. [Google Scholar] [CrossRef]
- Iglesias, E.J.; Mitschker, F.; Fiebrandt, M.; Bibinov, N.; Awakowicz, P. In Situ Measurement of VUV/UV Radiation from Low-Pressure Microwave-Produced Plasma in Ar/O2 Gas Mixtures. Meas. Sci. Technol. 2017, 28, 085501. [Google Scholar] [CrossRef]
- Fantz, U.; Briefi, S.; Rauner, D.; Wünderlich, D. Quantification of the VUV Radiation in Low Pressure Hydrogen and Nitrogen Plasmas. Plasma Sources Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]






| Fungi | Control | 30 s CPT | 60 s CPT | 120 s CPT | 180 s CPT |
|---|---|---|---|---|---|
| AA | 3.57 ± 0.1 a | 3.21 ± 0.1 a | 0.77 ± 0.8 b | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| AF | 4.18 ± 0.1 a | 3.01 ± 0.1 b | 0.67 ± 0.7 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| AN | 4.41 ± 0.0 a | 1.33 ± 0.7 b | 1.33 ± 0.7 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| CL | 3.98 ± 0.0 a | 2.43 ± 0.1 b | 0.67 ± 0.7 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| EN | 3.91 ± 0.1 a | 3.62 ± 0.0 a | 1.33 ± 0.7 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| FF | 6.34 ± 0.1 a | 5.85 ± 0.1 b | 4.99 ± 0.0 c | 3.70 ± 0.0 e | 4.0 ± 0.1 d |
| FG | 3.58 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| FO | 5.68 ± 0.0 a | 5.44 ± 0.0 b | 4.50 ± 0.0 c | 3.96 ± 0.0 d | 2.46 ± 0.1 e |
| FP | 5.94 ± 0.1 a | 5.70 ± 0.1 a | 4.78 ± 0.0 b | 2.80 ± 0.1 c | 2.93 ± 0.1 c |
| FS | 5.27 ± 0.0 a | 4.40 ± 0.0 b | 3.99 ± 0.0 c | 2.26 ± 0.1 e | 2.71 ± 0.1 d |
| Treatment | Germination Rate [%] |
|---|---|
| Control | 83.0 ± 4.1 a |
| 30 s CPT | 72.0 ± 3.4 b |
| 60 s CPT | 55.0 ± 5.2 c |
| 120 s CPT | 8.0 ± 3.4 d |
| 180 s CPT | 0.0 ± 0.0 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mravlje, J.; Kobal, T.; Regvar, M.; Starič, P.; Zaplotnik, R.; Mozetič, M.; Vogel-Mikuš, K. The Sensitivity of Fungi Colonising Buckwheat Grains to Cold Plasma Is Species Specific. J. Fungi 2023, 9, 609. https://doi.org/10.3390/jof9060609
Mravlje J, Kobal T, Regvar M, Starič P, Zaplotnik R, Mozetič M, Vogel-Mikuš K. The Sensitivity of Fungi Colonising Buckwheat Grains to Cold Plasma Is Species Specific. Journal of Fungi. 2023; 9(6):609. https://doi.org/10.3390/jof9060609
Chicago/Turabian StyleMravlje, Jure, Tanja Kobal, Marjana Regvar, Pia Starič, Rok Zaplotnik, Miran Mozetič, and Katarina Vogel-Mikuš. 2023. "The Sensitivity of Fungi Colonising Buckwheat Grains to Cold Plasma Is Species Specific" Journal of Fungi 9, no. 6: 609. https://doi.org/10.3390/jof9060609
APA StyleMravlje, J., Kobal, T., Regvar, M., Starič, P., Zaplotnik, R., Mozetič, M., & Vogel-Mikuš, K. (2023). The Sensitivity of Fungi Colonising Buckwheat Grains to Cold Plasma Is Species Specific. Journal of Fungi, 9(6), 609. https://doi.org/10.3390/jof9060609