Hydrogels and Wound Healing: Current and Future Prospects
Abstract
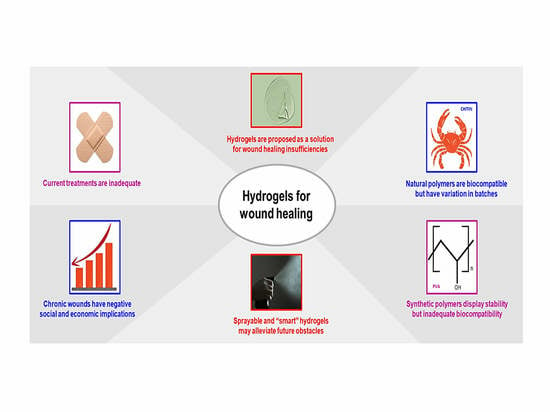
:1. Introduction
2. The Skin
3. Wound Healing Phases
4. Acute and Chronic Wounds
5. Socio-Economic Impact of Chronic Wounds
6. Current Treatment Methods
6.1. Dressings
6.2. Negative Pressure Therapy
6.3. Surgery
6.4. Hyperbaric Oxygen Therapy
7. Ideal Wound Healing System
8. Hydrogels in Wound Healing
9. Hydrogels as an Extracellular Matrix
10. Hydrogels for Treatment of Burn Wounds
11. Natural and Synthetic Hydrogels
11.1. Natural Hydrogels
11.1.1. Chitosan
11.1.2. Gelatin
11.1.3. Hyaluronic Acid
11.1.4. Alginate
11.2. Synthetic Hydrogels
11.2.1. Polyethylene Glycol (PEG)
11.2.2. Polyvinyl Alcohol (PVA)
11.2.3. Polyvinylpyrrolidone (PVP)
12. Advanced Hydrogels
12.1. Sprayable Hydrogels
12.2. “Smart” Hydrogels
13. Alternative Gels for Wound Healing
13.1. Nanogels
13.2. Aerogels
13.3. Cryogels
14. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, Y.; Zhang, H.; Zhu, T.; Chen, S.; Li, J.; Du, J.; Yan, X. A multifunctional chitosan composite aerogel based on high density amidation for chronic wound healing. Carbohydr. Polym. 2023, 321, 121248. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually cross-linked injectable hydrogels of poly (ethylene glycol) and poly [(2-dimethylamino) ethyl methacrylate]-b-poly (N-isopropyl acrylamide) as a wound healing promoter. J. Mater. Chem. B 2017, 5, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal 2016, 10, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans cells—Programmed by the epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Dorgalaleh, A.; Bahraini, M.; Shams, M.; Parhizkari, F.; Dabbagh, A.; Naderi, T.; Fallah, A.; Fazeli, A.; Ahmadi, S.E.; Samii, A.; et al. Molecular basis of rare congenital bleeding disorders. Blood Rev. 2023, 59, 101029. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Percival, N.J. Classification of wounds and their management. Surgery 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Nagle, S.M.; Stevens, K.A.; Wilbraham, S.C. Wound assessment. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Zabaglo, M.; Sharman, T. Postoperative wound infection. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tham, K.W.; Lim, A.Y.L.; Baur, L.A. The global agenda on obesity: What does this mean for Singapore? Singapore Med. J. 2023, 64, 182–187. [Google Scholar] [CrossRef]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef]
- Baranoski, S. Choosing a wound dressing, part 1. Nursing 2008, 38, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Kantak, N.A.; Mistry, R.; Varon, D.E.; Halvorson, E.G. Negative Pressure Wound Therapy for Burns. Clin. Plast. Surg. 2017, 44, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Cheung, A.; Bogie, K. Pressure Ulcers. In Essentials of Physical Medicine and Rehabilitation, 4th ed.; Frontera, W.R., Silver, J.K., Rizzo, T.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 849–859. [Google Scholar] [CrossRef]
- Wenhui, L.; Changgeng, F.; Lei, X.; Baozhong, Y.; Guobin, L.; Weijing, F. Hyperbaric oxygen therapy for chronic diabetic foot ulcers: An overview of systematic reviews. Diabetes Res. Clin. Pract. 2021, 176, 108862. [Google Scholar] [CrossRef] [PubMed]
- Holloway, S.; Harding, K.G. Wound dressings. Surgery 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Bilici, C.; Can, V.; Nöchel, U.; Behl, M.; Lendlein, A.; Okay, O. Melt-Processable Shape-Memory Hydrogels with Self-Healing Ability of High Mechanical Strength. Macromolecules 2016, 49, 7442–7449. [Google Scholar] [CrossRef]
- Maaz Arif, M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Ullah Khan, R.; Awais, S.M.; Arif Butt, M. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, D.; Chen, K.; Chattopadhyay, A.; Henn, D.; Wu, W.; Noishiki, C.; Magbual, N.J.; Mittal, S.; Mermin-Bunnell, A.M.; Bonham, C.A.; et al. Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 660145. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Tyler, M.; Vojnovic, B.; Pleat, J.; Harris, A.; Furniss, D. Human model of burn injury that quantifies the benefit of cooling as a first aid measure. J. Br. Surg. 2019, 106, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.U.; Samudrala, P.K.; Mandal, B.B. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds. Adv. Healthcare Mater. 2018, 7, 1801092. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, Q.-W.; Chen, Q.; Cui, C.; Duan, S.; Kang, Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S. Biomedical polymers: Synthesis, properties, and applications. Sci. China Chem. 2022, 65, 1010–1075. [Google Scholar] [CrossRef]
- Sahariah, P.; Kontogianni, G.-I.; Scoulica, E.; Sigurjonsson, O.E.; Chatzinikolaidou, M. Structure-activity relationship for antibacterial chitosan carrying cationic and hydrophobic moieties. Carbohyd. Polym. 2023, 312, 120796. [Google Scholar] [CrossRef]
- Joseph, C.; Daniels, A.; Singh, S.; Singh, M. Histidine-tagged Folate-Targeted Gold Nanoparticles for enhanced transgene expression in Breast Cancer Cells in Vitro. Pharmaceutics 2022, 14, 53. [Google Scholar] [CrossRef]
- Akinyelu, J.; Oladimeji, O.; Daniels, A.; Singh, M. Folate-Targeted Doxorubicin Delivery to Breast and Cervical Cancer cells using a Chitosan-Gold Nano-delivery System. J. Drug Deliv. Sci. Technol. 2022, 67, 102978. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef]
- Mousavi, S.; Khoshfetrat, A.B.; Khatami, N.; Ahmadian, M.; Rahbarghazi, R. Comparative study of collagen and gelatin in chitosan-based hydrogels for effective wound dressing: Physical properties and fibroblastic cell behavior. Biochem. Biophys. Res. Commun. 2019, 518, 625–631. [Google Scholar] [CrossRef]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, M. Antimicrobial gelatin nanofibers containing silver nanoparticles. Fibers Polym. 2008, 9, 685–690. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Q.; Chen, Y.; Xu, L.; Feng, M.; Xiong, Z.; Li, J.; Ren, J.; Liu, J.; Liu, B. Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication and accelerated chronic wound repair. Acta Pharm. Sin. B 2023, 13, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, F.; Longobardo, G.; Fabozzi, A.; di Gennaro, M.; Borzacchiello, A. Hyaluronic Acid-Based Wound Dressing with Antimicrobial Properties for Wound Healing Application. Appl. Sci. 2022, 12, 3091. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; Liang, Y.; Pan, G.; Guo, B. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Sanchez, M.F.; Guzman, M.L.; Apas, A.L.; Alovero, F.d.L.; Olivera, M.E. Sustained dual release of ciprofloxacin and lidocaine from ionic exchange responding film based on alginate and hyaluronate for wound healing. Eur. J. Pharm. Sci. 2021, 161, 105789. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Jang, J.; Seol, Y.-J.; Kim, H.J.; Kundu, J.; Kim, S.W.; Cho, D.-W. Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering. J. Mec. Behav. Biomed. Mater. 2014, 37, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloid. Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.K.; Parekh, S.H.; Dortdivanlioglu, B.; Rosales, A.M. Synthetic hydrogels as blood clot mimicking wound healing materials. Prog. Biomed. Eng. 2021, 3, 042006. [Google Scholar] [CrossRef] [PubMed]
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef]
- Chen, S.L.; Fu, R.H.; Liao, S.F.; Liu, S.P.; Lin, S.Z.; Wang, Y.C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transplant. 2018, 27, 275–284. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Martínez-Barbosa, M.E. A freeze-thawing method to prepare chitosan-poly (vinyl alcohol) hydrogels without cross-linking agents and diflunisal release studies. J. Vis. Exp. 2020, 14, e59636. [Google Scholar] [CrossRef]
- Muchová, M.; Münster, L.; Capáková, Z.; Mikulcová, V.; Kuřitka, I.; Vícha, J. Design of dialdehyde cellulose cross-linked poly (vinyl alcohol) hydrogels for transdermal drug delivery and wound dressings. Mater. Sci. Eng. C 2020, 116, 111242. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Wang, F.; Fan, Z.; Wang, H.; Tao, C.; Wang, Z. Preparation of polyurethane/polyvinyl alcohol hydrogel and its performance enhancement via compositing with silver particles. RSC Adv. 2017, 7, 46480–46485. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Wang, Y.; Zhou, G.; Ge, K.; Jiang, L. Adhesive, antibacterial and double cross-linked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing. Int. J. Biol. Macromol. 2023, 228, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Irmukhametova, G.S.; Shaikhutdinov, E.M.; Rakhmetullayeva, R.K.; Yermukhambetova, B.B.; Ishanova, A.K.; Temirkhanova, G.; Mun, G.A. Nanostructured Hydrogel Dressings on Base of Crosslinked Polyvinylpyrrolidone for Biomedical Application. Adv. Mater. Res. 2014, 875–877, 1467–1471. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Mirmasoudi, S.S.; Pourmohammadi-Bejarpasi, Z.; Feizkhah, A.; Mobayen, M.; Hedayati, M.; Sadeghi, M.; Esmailzadeh, M.; Mirkatoul, F.B.; Jamshidi, S. Polyacrylic acid/ polyvinylpyrrolidone hydrogel wound dressing containing zinc oxide nanoparticles promote wound healing in a rat model of excision injury. Heliyon 2023, 9, e19230. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, Y.; Chen, Z.; Yu, Y.; Wang, H.; Lu, X.; Zhao, J.; Wang, S. Chitosan-based multifunctional hydrogel for sequential wound inflammation elimination, infection inhibition, and wound healing. Int. J. Biol. Macromol. 2023, 235, 123847. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef]
- Tan, Y.; Cai, B.; Li, X.; Wang, X. Preparation and Application of Biomass-based Sprayable Hydrogels. Paper Biomater. 2023, 8, 1–19. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart bandages: The future of wound care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Khan, B.; Arbab, A.; Khan, S.; Fatima, H.; Bibi, I.; Chowdhry, N.P.; Ansari, A.Q.; Ursani, A.A.; Kumar, S.; Hussain, J.; et al. Recent progress in thermosensitive hydrogels and their applications in drug delivery area. MedComm—Biomater. Appl. 2023, 2, e55. [Google Scholar] [CrossRef]
- Dong, H.; Wang, L.; Du, L.; Wang, X.; Li, Q.; Wang, X.; Zhang, J.; Nie, J.; Ma, G. Smart Polycationic Hydrogel Dressing for Dynamic Wound Healing. Small 2022, 18, 2201620. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Nanogels for regenerative medicine. J. Control Release 2019, 313, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Dzulkharnien, N.S.F.; Rohani, R. A Review on Current Designation of Metallic Nanocomposite Hydrogel in Biomedical Applications. Nanomaterials 2022, 12, 1629. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.T.; Hussain, M.A.; Abbas, K.; Youssif, B.G.; Bashir, S.; Yuk, S.H.; Bukhari, S.N.A. Linseed hydrogel-mediated green synthesis of silver nanoparticles for antimicrobial and wound-dressing applications. Int. J. Nanomed. 2017, 12, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.; Alfallous, K.; Abogmaza, A. Antibacterial cellulose-based aerogels for wound healing application: A review. Biomed. Res. Ther. 2020, 7, 4032–4040. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, Z.; Hou, Y.; Jiang, H.; Li, Y.; Li, G.; Zhang, X. The Rising Aerogel Fibers: Status, Challenges, and Opportunities. Adv. Sci. 2023, 10, e2205762. [Google Scholar] [CrossRef]
- Abudula, T.; Colombani, T.; Alade, T.; Bencherif, S.A.; Memic, A. Injectable lignin-co-gelatin cryogels with antioxidant and antibacterial properties for biomedical applications. Biomacromolecules 2021, 22, 4110–4121. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, Y.; Chen, L.; Zheng, J.; Fan, X.J.; Xu, X.L.; Zhou, G.H.; Ullah, N.; Feng, X.C. An injectable antibacterial chitosan-based cryogel with high absorbency and rapid shape recovery for noncompressible hemorrhage and wound healing. Biomaterials 2022, 285, 121546. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.Y.; Liang, Y.P.; Yin, Z.H.; Chen, B.J.; Bai, L.; Han, Y.; Guo, B.L. Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- Cao, S.; Bi, Z.; Li, Q.; Zhang, S.; Singh, M.; Chen, J.D. Shape memory and antibacterial chitosan-based cryogel with hemostasis and skin wound repair. Carbohydr. Polym. 2023, 305, 120545. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Dey Chowdhury, S.; Babanejad, N. Cryogels: Advancing Biomaterials for Transformative Biomedical Applications. Pharmaceutics 2023, 15, 1836. [Google Scholar] [CrossRef] [PubMed]








| Type of Wound | Treatment | Function | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Infected | Gauze | Dries the wound | Removes necrotic tissue; used with topical products; can pack wounds | Adherence hinders healing. Frequent change in dressing needed. Secondary dressing necessary | [32,33,34] |
| High exudate | Foam | Absorbs high levels of exudates | Provides a moist environment. Easy to apply. Non-adherent | Adherence hinders healing. Unsuitable for eschar/non-draining wounds | [32,33,35] |
| Superficial skin disruption | Film | Allows for exchange of gases. | Stabilizes the wound site. Easy to visualize. Autolytic debridement | Damages new tissue. Poor moisture absorbance. Periwound maceration. | [32,33,36] |
| Eschar | Hydro- colloid | Absorbs high levels of exudates | Provides a moist environment. Insulation. Autolytic debridement. Is waterproof | Promotes granulated tissue. Unsuitable for infected wounds. | [25,32,33] |
| Product | Company | Constituent | Use |
|---|---|---|---|
| DermaSyn® | DermaRite Industries (NJ, USA) | Primary wound dressing with vitamin E | Partial and full-thickness chronic wounds |
| Neoheal® Hydrogel | Kikgel | Polyethylene glycol, polyvinylpyrrolidone, Agar, and 90% water | Low-exuding scabs, a abrasions, dry scabs, first, second-and third-degree burns, and ulcers |
| Restore Hydrogel | Hollister Inc. (IL, USA) | Gauze pad, Hyaluronic acid | Partial and full-thickness chronic wounds |
| ActivHeal® | Advanced Medical Solutions Ltd. (Oxon, UK) | Primary wound dressing with 85% water | Cavity wounds, pressure ulcers, diabetic foot ulcers, and leg ulcers |
| NU-GEL™ | Systagenix | Sodium alginate primary wound dressing | Diabetic foot ulcers, leg ulcers, venous ulcers |
| Purilon® | Coloplast | Calcium alginate, sodium carboxymethyl cellulose | Pressure ulcers, first and second degree burns, non-infected diabetic foot ulcers, leg ulcers |
| Simpurity™ Hydrogel | Safe n’ Simple | Acrylate, polyvinyl alcohol, polyethylene oxide, polyurethane | First and second-degree partial- thickness burns, low-exuding chronic wounds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. https://doi.org/10.3390/gels10010043
Gounden V, Singh M. Hydrogels and Wound Healing: Current and Future Prospects. Gels. 2024; 10(1):43. https://doi.org/10.3390/gels10010043
Chicago/Turabian StyleGounden, Varshan, and Moganavelli Singh. 2024. "Hydrogels and Wound Healing: Current and Future Prospects" Gels 10, no. 1: 43. https://doi.org/10.3390/gels10010043
APA StyleGounden, V., & Singh, M. (2024). Hydrogels and Wound Healing: Current and Future Prospects. Gels, 10(1), 43. https://doi.org/10.3390/gels10010043