2.1. Impedance Spectroscopy (IS) Characterisation and Modelling
The IS method has been employed to investigate the electrical behaviour of the fabricated devices. Capacitance and equivalent series resistance (ESR) have been obtained and analysed to assess the impact of the employed materials and their concentrations on the electrical and humidity-sensing performance.
Figure 1a,b illustrate the obtained bode plots of the fabricated devices at different relative humidity levels during the adsorption process.
Figure 1c,d compare the Nyquist and capacitance plots, respectively. In particular, capacitance (
C′) curves have been calculated from the following equation:
where
|Z| is the modulus and
Z″ is the imaginary part of the complex impedance.
Figure 1 depicts the impedance spectrum data obtained from PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors at various relative humidity (RH) levels, thereby exhibiting the trend of the electrical behaviour as a function of %RH and sensing layer composition.
Figure 1a compares the impedance modulus of the fabricated devices at different relative humidity levels. As can be noticed, all the capacitors exhibit a similar impedance modulus behaviour, thereby revealing two distinct trends. The first trend is characterised by a drop in the impedance modulus, with a slope of −1 from 0.1 Hz to a specific frequency, indicative of a capacitive behaviour. After this particular frequency, the impedance modulus flattens, exhibiting a slope equal to 0 and tending towards a particular value, revealing a transition from a capacitive to a resistive behaviour. Therefore, the frequency at which the impedance modulus changes its slope acts as a threshold frequency. This behaviour change can also be observed in
Figure 1b, where, between a frequency of 0.1 Hz and the specific threshold frequencies, the fabricated devices show phase values smaller than −45°, which are attributed to a capacitive behaviour. Beyond these threshold frequencies, the phase responses increase, with their values approaching 0°, indicative that at high frequencies, resistive behaviour predominates. The second trend observed relies on the variation in the impedance modulus in response to changes in humidity levels. This trend is characterised by a reduction in the impedance modulus values with increasing relative humidity. This finding is also evident in
Figure 1c, where the Nyquist plots intersect with the real impedance axis at lower values. This phenomenon can be attributed to an enhancement of the charge contribution due to increased charge carrier density. A higher density of charge carriers may result in increased capacitance values. Consequently, the capacitance spectres also reveal increased values in response to a humidity increase (
Figure 1d). This behaviour is widely exhibited by capacitive humidity sensors [
16,
33,
34].
The overall trend exhibited in
Figure 1 can be fitted according to the Cole–Cole model as an equivalent circuit (
Figure 2). In particular,
Figure 1c compares the obtained Nyquist plots, wherein part of a characteristic semi-circular shape is exhibited by these curves, revealing a capacitor and resistor connected in parallel. The semi-circular shape represents the capacitive behaviour of the fabricated composite and non-composite ILGPE-based capacitors. Nevertheless, at high frequencies, the semi-circular responses present some shifts from the origin, indicating the existence of a resistance in series with the parallel combination. This deviation is typically ascribed to the bulk resistance of the materials that comprise the capacitors, demonstrating that two sub-circuits connected in series form the equivalent circuit.
The first sub-circuit within the equivalent circuit model comprises a series resistor (
Rs). Meanwhile, the other circuit involves the parallel combination of a constant phase element (
CPE) and a resistor (
Rp) related to a leakage resistance. The CPE is a frequency-dependent element that simulates the formation of a double-layer capacitance. The
CPE appears due to the charge accumulation at the electrode–electrolyte interfaces, as well as at the boundaries of the nanoparticles. This element can be defined by Equation (2).
QCPE represents the admittance of an ideal capacitance, and
is the parameter employed to model the frequency dispersion resulting from non-ideal capacitive behaviour owing to factors such as electrode roughness or electrolyte inhomogeneity [
35,
36].
The
Rs represents the ESR, which describes the combination of the internal resistive effects of the materials that compose a capacitor. Therefore,
Rs arises from the bulk resistance of the electrolyte and the electrodes internal resistance [
36]. Specific frequency ranges have been selected to illustrate a portion of the Nyquist plots (
Figure 1c) to appreciate each device’s exhibited ESR values at different %RH levels. The
Rs values have been estimated by determining the intersections of the Nyquist curves with the x-axis at higher frequencies [
35,
36].
2.3. Humidity Sensor Performance
The humidity impedimetric and capacitive responses of the different fabricated ILGPE- and SiO
2 CILGPE-based capacitors have been evaluated at various frequencies in a relative humidity range from 20 %RH to 90 %RH. Seven different %RH levels have been applied to test the impedance and capacitance responses during the adsorption and desorption processes. The responses of three PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors are displayed in
Figure 4.
As illustrated in
Figure 4, the impedance responses of the fabricated humidity sensors exhibit a decreasing trend with relative humidity; meanwhile, the capacitance responses reveal an increasing trend due to the relative humidity increase. The exhibited impedance and capacitance behaviours could be ascribed to the enhancement of ion conduction resulting from the interaction of the sensing layer with water molecules. Higher relative humidity levels imply an increase in the probability of the adsorption of water molecules and, hence, an increase in the charge carrier density. As a result, the developed capacitors’ impedance diminishes, and capacitance rises. Regarding the role of the operating frequency, it can be noticed that the impedance and capacitance responses tend to exhibit lower variations at high frequencies, indicating a decrease in the sensitivity of the ILGPE and SiO
2 CILGPE capacitors with the frequency. This effect can also be ascribed to water molecules’ polarisation dynamics. Adsorbed water molecules can follow changes in the electric field at low frequencies. However, they are not able to follow faster electric field changes that occur at high frequencies [
10,
16].
Furthermore, it is noticeable that the PVA:PVP0% capacitors reveal linear impedance responses to humidity changes. However, adding SiO2 nanoparticles, PVP, or both stimulates the interaction with water molecules, leading to impedance responses displaying non-linear behaviours. This observation reduces the viability of using impedance modulus as a humidity-sensing parameter since one of the desired sensor characteristics is linearity. Consequently, evaluations of sensitivity and hysteresis have been exclusively focused on the capacitance responses.
Following the analysis of the exhibited humidity-sensing responses at different frequencies, the capacitance sensitivities of the sensors to changes in relative humidity in the ambient chamber at 25 °C have been calculated using Equation (3), as typically used [
1,
13,
34,
40,
41]:
where,
and
are the capacitance values at 90% and 20% of relative humidity, respectively.
Figure 5 compares the sensitivity values vs. the evaluated frequencies and shows an evident impact on capacitance sensitivities because of the addition of PVP and SiO
2 nanofillers to the PVA-based ILGPE. The exhibited capacitance responses indicate that using PVP and SiO
2 nanoparticles is supportive in driving the sensitivity at the evaluated frequencies. Different hydrophilic properties mean distinct ways to interact and react with water molecules. In particular, in
Figure 5, SiO
2 PVA:PVP
0% and SiO
2 PVA:PVP
25% capacitors exhibit higher capacitance sensitivities than the PVA:PVP
0% capacitor, thereby revealing the positive impact of the charge pathways provided by the nanoparticles on the sensitivity.
Figure 5 also reveals that incorporating PVP and SiO
2 into the PVA-ILGPE increases the capacitance sensitivity within the frequency range of 1 kHz to 100 kHz. These findings could be ascribed to the number of active sites per volume available in the sensing layer. Firstly, the presence of PVP in the sensing layer composition implies a relevant change in the available active sites per volume in the sensing layer because of its amorphous nature. Secondly, the presence of SiO
2 nanoparticles affects the sensitivity since SiO
2 fillers can increase the available surface area and number of active sites per volume [
1]. This effect could arise from the reduction in the crystallinity of the ILGPEs, which is supported by the findings revealed in
Figure 3, and the high area-to-volume ratio of the nanoparticles available for ion exchanges. Finally, it is noteworthy that the device that exhibits the highest capacitance sensitivities is the SiO
2 PVA:PVP
25% capacitor, revealing the positive contribution of the combination of the SiO
2 and PVP benefits. This result validates the hypothesis that incorporating SiO
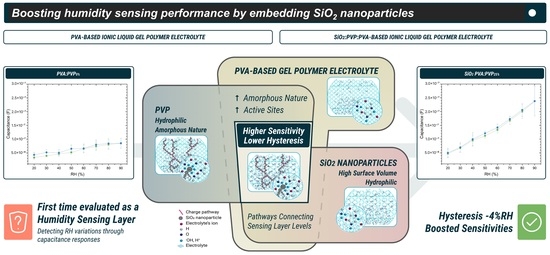
2 nanobeads and PVP can stimulate a reduction in the degree of crystallinity of PVA. Therefore, the resultant electrolyte exhibits a more amorphous layer with a rougher surface, increasing the sensing area and enhancing the sensitivities.
In addition to the analysis of the impact of PVP and SiO
2 nanoparticles on the sensitivity, the hysteresis parameter has also been analysed. Hysteresis refers to the highest deviation between the obtained ILGPE and SiO
2 CILGPE capacitor responses during desorption and adsorption processes. Factors such as porous structures or surface morphology of the sensing layer mainly dominate the hysteresis parameter [
42]. Therefore, hysteresis is a significant parameter in evaluating the sensing performance and understanding the absorption principle of the humidity sensor. Initially, to obtain the adsorption responses, the capacitors were exposed to a controlled environment in which the RH was gradually increased from 20 %RH to 90 %RH over a specific time. Afterwards, to measure the desorption responses, the relative humidity was progressively decreased from 90 %RH to 20 %RH. The impedance spectra of the ILGPE and SiO
2 CILGPE capacitors were obtained at each relative humidity variation. After calculating the capacitance values at each specific %RH during the adsorption and desorption processes, the hysteresis value was calculated using the following Equation (4) [
1,
13]:
where
and
are the obtained capacitance values during desorption and adsorption at the steady humidity level, respectively, and S is the sensitivity.
Figure 6 compares the hysteresis characteristics exhibited by the fabricated devices at the evaluated frequencies.
As is noticeable from
Figure 6, SiO
2 PVA:PVP
0% and SiO
2 PVA:PVP
25% capacitors exhibit lower hysteresis values than their electrolyte counterparts without embedded SiO
2 nanoparticles in the electrolyte. This finding indicates that SiO
2 CILGPE capacitors are more susceptible to humidity changes than ILGPE-based capacitors, exposing the dominant role of silica nanoparticles in determining the hysteresis. SiO
2 nanofillers could generate pathways that can facilitate the diffusion of water molecules since they indicate a connection between the different levels of the sensing layer, thereby promoting water molecule diffusion. Furthermore, it is essential to highlight the constructive contribution of PVP in SiO
2 CILGPE capacitors. The simultaneous existence of SiO
2 nanobeads and PVP in the PVA-based ILGPE capacitor exhibits the lowest hysteresis values in this study, revealing the favourable combination of these materials. The reduction in hysteresis may be primarily attributed to the amorphous nature of PVP. The inherent amorphous structure of PVP disrupts the crystallinity of the PVA matrix, increasing the amorphousness of the SiO
2 CILGPE and leading to a reduction in zones with a crystalline phase that can hinder the diffusion of water molecules, thus facilitating their transport. Moreover, the hydrophilicity of PVP stimulates the formation of additional hydrogen bonds with water, affecting the retention and release of water molecules and increasing the susceptibility of SiO
2 CILGPE to %RH variations. These findings are consistent with those of [
16,
42], which indicate that high surface-to-volume-ratio materials result in excellent humidity-sensing performance, although their intrinsic chemical and physical characteristics are more susceptible to changes, which directly influence the hysteresis.
As a summary,
Figure 7 depicts the capacitance responses of the PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors during the adsorption and desorption processes at the frequencies that exhibit higher sensitivities and lower hysteresis values. Consequently, for PVA:PVP
0% capacitors, the optimal working frequency is 50 kHz, while for SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors, it is 10 kHz. Notably, the PVA:PVP
0% ILGPE capacitor shows a capacitance response with a sensitivity of about 739 pF/%RH. As explained above, the incorporation of silicon dioxide nanoparticles stimulates increased sensibility and decreased hysteresis. As a result, the SiO
2 PVA:PVP
0% CILGPE capacitor exhibits capacitance sensitivities of about 1780 pF/%RH with a hysteresis value of 10.64 %RH.
Furthermore, adding PVP also enhances the sensing performance, leading to capacitance sensitivities of 1950 pF/%RH with maximum hysteresis of about 12.89 %RH. Finally, as is noticeable, the SiO2 PVA:PVP25%-ILGPE-based capacitor shows the highest capacitance sensitivity with the lowest hysteresis values, exhibiting capacitance sensitivities of 2730 pF/%RH with the lowest maximum hysteresis about 3.28 %RH, respectively, thereby revealing the positive effect of combining PVP and SiO2 fillers. The lowest hysteresis value can be mainly ascribed to the presence of SiO2 nanoparticles. As explained earlier, SiO2 nanoparticles may facilitate the diffusion of water molecules by connecting different levels of the sensing layer (top and bottom). In addition to this effect, the presence of PVP, which can increase the amorphousness, could reduce the barriers to water molecule movement. Irregular arrangements may provide more pathways for water molecule diffusion, which can reduce the hysteresis.
On the other hand, regarding the higher sensitivity, the coexistence of PVP and SiO2 nanoparticles increases the amorphousness of the sensing layer. Therefore, their presence can increase the surface area where water molecules can be adsorbed. Additionally, as both materials are hydrophilic, their coexistence further increases the active sites available for the interaction between water molecules and the sensing layer, increasing the susceptibility to humidity changes.
Additionally,
Table 1 provides an overview of the linearity performance exhibited by PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors at 10 kHz within the suggested detection range. The regression analysis conducted on ILGPE- and SiO
2 CILGPE-based capacitors demonstrates the existence of a linear relationship between the relative humidity and capacitance responses. The resulting high R-squared values show a reliable and accurate linear fit, further emphasising the linear performance within the proposed sensing range. Furthermore, regarding the detection limit, the criteria for selecting sensing limits is based on discarding the relative humidity levels that reveal differences lower than 3% compared to their previous %RH levels. Consequently, the PVA:PVP
0%-based capacitor is suggested to operate within the 20–70 %RH sensing range. From
Table 1, it can be concluded that the addition of SiO
2 and PVP could expand the humidity-sensing span, revealing the positive impact of these materials on the sensing range. Additionally, as the sensitivity can also be defined as the slope of the calibrated fitting response of a sensor [
1], this table also shows the sensitivity values of the calibrated responses.
As a result of the findings discussed above, it can be concluded that the most suitable electrolyte for acting as a humidity-sensing layer is the SiO2 PVA:PVP25% CILGPE in terms of sensitivity and hysteresis. Nevertheless, it is important to investigate the reliability of a sensor in the context of humidity sensing. To evaluate the reliability of the optimised SiO2 CILGPE, the long-term stability, the effect of the temperature on the capacitance response, and the impact of the cyclability on the hysteresis parameter have been investigated.
The cyclability of the optimised sensor has been examined since it is a crucial parameter in determining the reliability and durability of a sensor. Cyclability refers to the ability exhibited by a sensor to maintain its sensing performance over many cycles of usage. In particular, the cyclability has been analysed by measuring the adsorption and desorption responses for ten cycles to determine their impact on the hysteresis parameter. The study of the hysteresis parameter across several cycles provides a first insight into how consistent and reliable the capacitive humidity response can be over repeated cycles. Additionally, understanding how the cyclability affects the hysteresis can give information about the sensor’s durability and stability.
Figure 8 shows the obtained hysteresis over ten consecutive cycles, providing insights into the stability and repeatability of the optimised sensor. In particular,
Figure 8 illustrates that the capacitance response of a SiO
2 PVA:PVP
25% capacitor exhibits similar hysteresis during ten cycles, showing hysteresis values around 3.6 %RH. This finding may indicate that, in terms of hysteresis, the SiO
2 PVA:PVP
25% capacitor could exhibit a consistent and reliable humidity-sensing performance over ten cycles.
Furthermore, to analyse the long-term stability, a SiO
2 PVA:PVP
25%-based capacitor was exposed to natural environmental conditions for five months after its fabrication. A comparison between the responses of the SiO
2 PVA:PVP
25% five months after the fabrication process and in its initial state is displayed in
Figure 9a. This analysis provides initial insights into the ability of the SiO
2 PVA:PVP
25% CILGPE to maintain its sensitivity and hysteresis characteristics.
Figure 9a compares the initial and the five-month capacitance responses, exhibiting a humidity-sensing deterioration. After five months, the capacitance response shifts to higher values and displays a reduced sensitivity. Notably, the initial capacitance responses exhibit a calibrated sensitivity of 2140 pF/%RH, while five months later, the same device presents a reduction of about 50% in its sensitivity, thereby revealing a sensitivity of 1024 pF/%RH. However, despite this observed sensitivity degradation, the SiO
2 PVA:PVP
25% CILGPE-based capacitors still display a linear behaviour, thereby indicating the possibility of compensation for this degradation. This alteration in the humidity capacitance response indicates some concerns that merit further investigation. Therefore, it is advisable to delve deeper through additional research aimed at studying and mitigating this degradation.
Considering that temperature can impact humidity sensors’ long-term stability and durability, a five-month-old device has also been employed in temperature-dependence analysis. This approach provides insights into SiO
2 CILGPE-based capacitors’ performance and endurance under fluctuating thermal conditions, revealing more information about their reliability over time. As is widely known, most hygroscopic materials reveal variations in their characteristics under different temperatures, exhibiting a dependency on temperature. This effect is particularly noticeable in impedimetric and capacitance sensors. Therefore, the evaluation of how temperature affects humidity-sensing responses may result in the mitigation of its impact. Thus, the reliability of humidity sensors can be improved by compensating for the potential erroneous humidity readings. To determine the effect of the temperature variation on the humidity-sensing performance of the optimised sensing layer, IS measurements of RH at three different temperatures (25 °C, 35 °C, and 45 °C) have been performed at the selected working frequency.
Figure 9b illustrates the resulting capacitive humidity responses at the proposed working frequency of 10 kHz under different temperatures. Notably,
Figure 9b shows that the obtained capacitance responses of the optimised sensing layer are shifted to higher values with the increase in ambient temperature. This behaviour has been previously reported and indicates that an increase in temperature can provide additional energy to charge carriers, leading to a stronger thermal movement of the water molecules [
13,
43].
As a consequence, there is an increase in the charge carriers’ mobility, which can also increase the capacitance values. Furthermore, as can be noticed in
Figure 9b, the obtained capacitance responses at different temperatures depict similar linear trends without exhibiting significant sensitivity variations. This finding indicates a suitable temperature dependence to function as a humidity sensor since the deviation change promoted by the temperature increase can be easily compensated.
Finally,
Figure 10 and
Table 2 compare the SiO
2 PVA:PVP
25% capacitors’ sensing properties with those previously exhibited in the SOA.
Figure 10 shows that, when compared with some recently reported capacitive humidity sensors based on pyromellitic dianhydride (PMDA)-oxydianiline (ODA)/TiO
2 [
13], polyaniline (PANI)/1% Cu–ZnS [
33], poly(ionic liquid) [
44], PPy/graphene oxide (GO) [
9], graphene oxide quantum dots (GOQD)/Nafion [
11], PVA/cellulose acetate butyrate (CAB) [
6], keratine/1% carbon fibres (CF) [
10], graphene–carbon nanotube (CNT)–silicone adhesive (SA) [
34], PVDF-BaTiO
3 [
15], GO-doped poly(vinylidene fluoride-trifluoroethylene) (P(VDF-TrFE))/lithium chloride (LiCl) [
41], or PVP+CAB [
8], the SiO
2 PVA:PVP
25%-CILGPE capacitor has relevant detection parameters. These prior studies combine nanofillers or salts with polymers or use ILs to enhance sensitivity and hysteresis in humidity sensors. For instance, incorporating carbon fibres into a keratin matrix resulted in a capacitance sensitivity of 633.12 pF/%RH across a wide sensing range. Similarly, the PPy and graphene oxide composite yielded a low-hysteresis humidity sensor with a sensitivity of 1670.3 pF/%RH. Furthermore, the polymerisation of an ionic liquid or adding salt into a PVA matrix to create microstructures revealed capacitance sensitivities of 1.8 nF/%RH with low hysteresis and 1.41 pF/%RH, respectively.
In contrast, this study explores the impact of silica nanoparticles, PVP, and their combination on an ILGPE, revealing the beneficial effects of the simultaneous presence of these materials and exhibiting a higher calibrated capacitance sensitivity with a value of 2660 pF/%RH and low hysteresis. Therefore, this work introduces a novel composite that blends polymers, nanofillers, and an ionic liquid. The resulting composite is based on eco-friendly materials and uses an easy and fast fabrication process. This approach exhibits an enhancement of capacitance sensitivity and hysteresis. Finally, as could be concluded from
Table 2 and
Figure 10, the SiO
2 PVA:PVP
25%-CILGPE-based device outperforms some reported capacitive humidity sensors in terms of sensitivity. Approximately 64% of the studies summarised in the table reported capacitance sensitivities below 1500 pF/%RH. The good sensitivity and hysteresis of the SiO
2 PVA:PVP
25%-CILGPE-based capacitor, combined with its simple and cost-effective fabrication process and possible easy compensation of its temperature dependence, position the optimised device as a viable option for a humidity sensor.
2.4. Humidity-Sensing Mechanism
Previous research has established a link between the electrical and physical behaviours of humidity sensors by fitting their complex impedance spectroscopy data using equivalent circuits, allowing for the investigation of the sensing mechanism [
9,
16,
43]. Consequently, to investigate the working mechanism of the PVA:PVP
0%, SiO
2 PVA:PVP
0%, and PVA:PVP
25% capacitors and the impact of the addition of SiO
2 nanoparticles, PVP, or both on the sensing mechanism, the variation in the values of the elements that compose the equivalent circuit have been analysed as a function of %RH. As previously explained, the obtained impedance spectra can be modelled using an equivalent circuit. This electrical circuit consists of a resistance (
Rs) in series with the parallel combination of a
CPE and a leakage resistance (
Rp). The
Rs parameter corresponds to the bulk resistance of the electrolyte and the electrodes, while the
CPE is attributed to the double-layer capacitances. Alterations in humidity mainly affect the
CPE, which is noticeable in the
QCPE parameter and the value of
Rs. Consequently, the impact of moisture on both parameters has been analysed, as any changes in the electrical characteristics of the electrolyte are reflected in these parameters.
On the one hand,
Figure 11 shows the evident tendency followed by the values of
Rs and
QCPE with changes in humidity. Notably,
Figure 11a illustrates the obtained
Rs values for PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors across different %RH levels, exhibiting their respective trends. As displayed in
Figure 11a, there is an evident reduction in
Rs values as humidity increases. This finding aligns with the impedance modulus trend and indicates an enhancement in the charge conduction that reduces the electrolyte resistance. This improved charge conduction could be the result of an increase in the charge content and enhanced ionic mobility. The higher ion density can be attributed to the ability of PVA, PVP, and SiO
2 nanoparticles to absorb and interact with water molecules from the environment. This effect is also noticeable in the behaviour of the
QCPE parameter relative to relative humidity levels.
Figure 11b shows that
QCPE values increase with rising humidity. This behaviour is widely exhibited by capacitive humidity sensors [
16,
33,
34]. The higher
QCPE values due to the %RH increase also indicate an enhancement in charge density, thereby promoting the formation and enhancement of electrical double-layer capacitance.
Therefore, the humidity-sensing mechanism works as depicted in
Figure 12. Initially, at low %RH levels, an initial interaction transpires between the surface of the sensing layer and the water molecules present in the environment, leading to some water molecules being trapped at the outermost levels of the sensing layer (
Figure 12a,b). This first interaction forms a chemisorbed layer formed by hydrogen bonds between water molecules and the hydroxyl groups in the sensing material, as explained in [
1,
20,
45]. This interaction is mainly attributed to the inherent hydrophilicity of the sensing layer, which has a minor impact on the charge conduction since chemisorbed molecules are not free to move [
1]. As the humidity rises, the sensing layer transitions from chemisorption to physisorption interactions. At this point, the physically adsorbed water molecule layers add to the chemically adsorbed layer, forming a continuous water molecule layer. These additional water molecules start to contribute to the charge conduction of the sensing layer due to the conduction of some extra ions [
20,
45]. This process leads to a decrease in the
Rs parameter and an increase in the
QCPE values. With further increases in the humidity, a fraction of the confined water molecules on the most superficial levels start to penetrate the polymeric layer, revealing the existence of an internal–external density balance of water molecules until a state of equilibrium is reached (
Figure 12c). At this point, the electrical behaviour is mainly controlled by charge conduction, resulting in even lower
Rs and higher
QCPE values. This finding is attributed to improved charge conduction due to (i) enhanced charge mobility, as the presence of continuous water molecules may allow higher mobility of the ions in the sensing layer, and (ii) a higher effective charge density, owing to the extra ions (H
3O
+, H
−) contributing to the conduction. Consequently, higher relative humidity levels imply an increase in charge density inside the sensing layer, reducing the impedance of the polymer electrolyte and boosting capacitance values (
Figure 12d,e).
In contrast, during a decrease in %RH, this internal–external density balance is disrupted, and the trapped water molecules inside the sensing layer try to escape adsorption and initiate diffusion. This diffusion process occurs gradually, commencing with freeing the trapped water molecules at the outermost levels. Subsequently, the trapped molecules at the deeper layers start to ascend and diffuse, attaining equilibrium, decreasing the number of charges precedent from the water molecules’ interactions, increasing the impedance, decreasing the capacitance, and, hence, increasing the Rs and decreasing the QCPE values.
This proposed sensing mechanism is consistent with those discussed in [
10,
16,
20,
33,
40,
42,
45]. These previous studies explain that the humidity-sensing mechanism of polymeric sensors is governed by the interaction between the humidity-sensitive layer and water molecules, which is mainly explained by chemisorption and physisorption processes, as well as Grotthus chain reactions. The hydrophilic polymer possesses active sites that facilitate the interaction with water molecules, promoting an increase in the number of charge carriers within the host polymer.
On the other hand,
Figure 11a,b also illustrate the impact of the addition of SiO
2, PVP, and both materials to the PVA-based ILGPE on the sensing mechanism. Firstly,
Figure 11a displays how the composition of the sensing layer affects the
Rs behaviour at different %RH levels. In particular, as %RH increases from 20 to 90%, the
Rs values for PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors ranged from 34 Ω, 51 Ω, 52 Ω, and 148 Ω to 17 Ω, 19 Ω, 20 Ω, and 21 Ω, respectively. Notably, incorporating PVP and SiO
2 fillers leads to higher
Rs values. Higher
Rs values could suggest a hindrance to charge transport at lower humidity levels, indicating that charge carriers require more energy to be transported within the polymer electrolyte [
12]. This effect is most pronounced at low humidity levels, where PVP and SiO
2 nanoparticles may attempt to retain the charge due to their hydrophilic properties, impeding the mobility of some charge carriers.
Secondly,
Figure 11b shows the evolution of the
CPE element with the humidity, depending on the composition of the electrolyte, through the
QCPE parameter. At 20 %RH and 90 %RH, the PVA:PVP
0%, SiO
2 PVA:PVP
0%, PVA:PVP
25%, and SiO
2 PVA:PVP
25% capacitors exhibit
QCPE values range from 0.32 μF, 0.34 μF, 0.39 μF, and 0.34 μF to 0.39 μF, 0.45 μF, 0.45 μF, and 0.42 μF, respectively. This trend aligns with the abovementioned capacitance responses. The higher
QCPE values may be ascribed to the amorphousness caused by adding PVP and SiO
2 nanoparticles. Additionally, the higher variations in
QCPE values with increasing humidity can also be attributed to the expanded air-contact area on the surface of the sensing layer, enhancing the sensitivity of the devices. This hypothesis is consistent with the results from the surface topography analysis and the observed capacitance responses.
In summary, the results exhibit that adding PVP to a PVA-based gel polymer electrolyte leads to higher capacitance values in response to humidity variation (
Figure 11b). This effect could be ascribed to PVP’s amorphous nature and hydrophilic properties. Firstly, the excellent miscibility between PVP and PVA due to hydrogen bonding formation, coupled with the amorphousness of PVP, decreases the degree of crystallinity of the PVA matrix [
37,
38,
39]. The reduction in the crystalline phases and increase in the amorphous phase enhance the ionic conductivity of the ILGPE [
36], which can stimulate a capacitance Increase. Secondly, the amorphousness induced by PVP increases the number of available active sites per volume in the sensing layer. This, combined with the hydrophilic nature of PVP, may enhance the interaction between the sensing layer and the water molecules, leading to a higher charge density within the electrolyte layer and, hence, higher variations in the capacitance (
Figure 12g,h).
Moreover, SiO
2 CILGPE capacitors show more significant changes in
QCPE values than ILGPE-based capacitors. This effect can be mainly attributed to the active sites for ion exchange provided by SiO
2, which modifies the interaction of charge carriers with the sensing layer. As revealed in [
20], embedding SiO
2 nanoparticles into a polymer matrix offers several beneficial effects, including an increased charge contribution owing to their hydrophilic nature and the creation of new effective charge transport pathways, among others. These effects provided by the presence of SiO
2 nanoparticles can contribute to the overall sensing mechanism. Incorporating nanoparticles in the hydrophilic polymer matrix enhances the available surface area for water interaction, increasing the amount of water molecules adhered to the sensing layer. This is achieved by introducing active sites that stimulate water adsorption [
1,
16], leading to improved binding of water molecules. Consequently, the probability of capturing water molecules increases, resulting in an augmented charge density within the humidity-sensitive layer.
Furthermore, as depicted in
Figure 12f,h, the addition of silicon dioxide nanoparticles can introduce nanostructures that establish connections between different levels of the sensing layer. These silica structures can act as pathways for charge carriers and water molecules, facilitating their movement through the sensing layer. Consequently, these nanostructures can play a crucial role during desorption, providing a path for water molecules to traverse between the bottom and surface of the humidity-detection layer, assisting in the diffusion of water molecules into the environment and enhancing the water molecules’ equilibrium. This structural effect can improve the responsiveness of the humidity sensing, leading to a reduction in the hysteresis parameter. This hypothesis is consistent with the results shown in the analysis of the hysteresis parameter.