Chitosan–Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties
Abstract
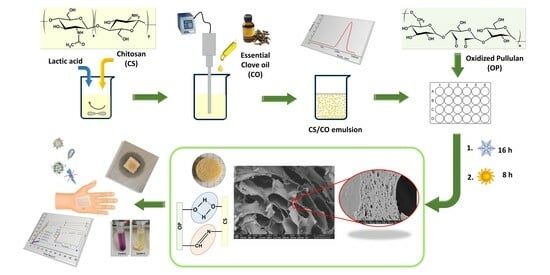
:1. Introduction
2. Results and Discussion
2.1. Preparation and Optimization of CS/CO Emulsion
2.2. Synthesis and Characterization of CS/OP Hydrogels with and without CO
2.2.1. FT-IR Spectra
2.2.2. Morphology and Porosity
2.2.3. Swelling Behavior
2.2.4. Mechanical Properties
2.3. In Vitro Release Studies
2.4. Antioxidant Activity
2.5. Antibacterial Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Oxidized Pullulan
4.3. Preparation of CO-Loaded Hydrogels
4.3.1. Preparation of CS/CO Emulsion
4.3.2. Preparation of CO-Loaded CS/OP Hydrogels
4.4. Determination of the Degree of Oxidation
4.5. Determination of Chemical Cross-Linking Degree by Ninhydrin Assay
4.6. Characterization
4.6.1. CS/CO Emulsion
4.6.2. CO-Loaded CS/OP Hydrogels
4.7. Swelling Behavior
4.8. Mechanical Properties
4.9. In Vitro Clove Oil Release Study
4.10. Antioxidant Activity
4.11. Antimicrobial Activity
4.11.1. MIC Determination of the CO
4.11.2. Difusimetric Test of Unloaded and CO-Loaded CS/OP Hydrogels
4.11.3. Time-Kill Studies
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Mazutti da Silva, S.M.; Rezende Costa, C.R.; Martins Gelfuso, G.; Silva Guerra, E.N.; De Medeiros Nóbrega, Y.K.; Gomes, S.M.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; Magalhães, P.O. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules 2019, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Tit, D.M.; Bungau, S.G. Antioxidant Activity of Essential Oils. Antioxidants 2023, 5, 383. [Google Scholar] [CrossRef] [PubMed]
- Yammine, J.; Chihib, N.E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in essential oils encapsulation: Development, characterization and release mechanisms. Polym. Bull. 2023, 81, 3837–3882. [Google Scholar] [CrossRef]
- Chen, L.-H.; Cheng, L.-C.; Doyle, P.S. Nanoemulsion-Loaded Capsules for Controlled Delivery of Lipophilic Active Ingredients. Adv. Sci. 2020, 7, 2001677. [Google Scholar] [CrossRef]
- Purwanti, N.; Zehn, A.S.; Pusfitasari, E.D.; Khalid, N.; Febrianto, E.Y.; Mardjan, S.S.; Kobayashi, A.I. Emulsion stability of clove oil in chitosan and sodium alginate matrix. Int. J. Food Prop. 2018, 21, 566–581. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Heuzey, M.-C. Chitosan-based conventional and pickering emulsions with long-term stability. Langmuir 2016, 32, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Pan, W.; Su, Y.; Yang, Y. Physical and Antimicrobial Properties of Thyme Oil Emulsions Stabilized by Ovalbumin and Gum Arabic. Food Chem. 2016, 212, 138–145. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. Int. J. Food Microbiol. 2016, 226, 20–25. [Google Scholar] [CrossRef]
- Ghosh, V.; Saranya, S.; Mukherjee, A.; Chandrasekaran, N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids Surf. B 2013, 105, 152–157. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Sui, Z.; Lu, W.; Corke, H. Soybean lecithin-stabilized oil-in-water (o/w) emulsions increase the stability and in vitro bioaccessibility of bioactive nutrients. Food Chem. 2021, 338, 128071. [Google Scholar] [CrossRef] [PubMed]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crops Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Huerta, R.R.; Silva, E.K.; El-Bialy, T.; Saldana, M.D.A. Clove essential oil emulsion-filled cellulose nanofiber hydrogel by high-intensity ultrasound technology for tissue engineering applications. Ultrason. Sonochem. 2020, 64, 104845. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Gao, Y.; Xie, Z.; Zhou, M.; He, Y.; Wu, H.; Zhou, W.; Dong, X.; Yang, Z.; et al. Cinnamon oil-loaded composite emulsion hydrogels with antibacterial activity prepared using concentrated emulsion templates. Ind. Crops Prod. 2018, 112, 281–289. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Q.; Li, Y.; Xu, R.; Li, R.; Wu, D.; Huang, R.; Yang, Z.; Li, Y. Hydrogel encapsulating wormwood essential oil with broad-spectrum antibacterial and immunomodulatory properties for infected diabetic wound healing. Adv. Sci. 2024, 11, 2305078. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Shen, X.; Xiao, D.; Rong, L.; Mao, Z.; Wang, B.; Sui, X.; Zhao, M.; Feng, X. Antibacterial thyme oil-loaded zwitterionic emulsion hydrogels. J. Mater. Chem. B 2022, 10, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Xu, Y.; Xu, G.; Pan, S. Sequence-selected C13-Dipeptide self-assembled hydrogels for encapsulation of Lemon Essential Oil with antibacterial activity. J. Agric. Food Chem. 2022, 70, 7148–7157. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Liu, Y.; Yue, Y.; Liu, Y.; Guo, F. Essential oil nanoemulsion hydrogel with anti-biofilm activity for the treatment of infected wounds. Polymers 2023, 15, 1376. [Google Scholar] [CrossRef]
- Catanzano, O.; Straccia, M.C.; Miro, A.; Ungaro, F.; Romano, I.; Mazzarella, G.; Santagata, G.; Quaglia, F.; Laurienzo, P.; Malinconico, M. Spray-by-spray in situ cross-linking alginate hydrogels delivering a tea tree oil microemulsion. Eur. J. Pharm. Sci. 2015, 66, 20–28. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Li, Y.; Li, Y.; Liu, H.; Yang, Z.; Wu, H.; Hu, Y. Formation of cinnamon essential oil/xanthan gum/chitosan composite microcapsules basing on Pickering emulsions. Colloid Polym. Sci. 2022, 300, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Low, W.L.; Kenward, M.A.K.; Amin, M.C.I.M.; Martin, C. Ionically Crosslinked Chitosan Hydrogels for the Controlled Release of Antimicrobial Essential Oils and Metal Ions for Wound Management Applications. Medicines 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.G.; Nita, L.E.; Rosca, I.; Croitoru, A.; Ghilan, A.; Tartau-Mititelu, L.; Grigoras, A.V.; Cretu, B.-E.-B.; Chiriac, A.P. Alginate-based hydrogels enriched with lavender essential oil: Evaluation of physicochemical properties, antimicrobial activity, and in vivo biocompatibility. Pharmaceutics 2023, 15, 2608. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 751062. [Google Scholar] [CrossRef]
- dos Santos, M.K.; Kreutz, T.; Danielli, L.J.; De Marchi, J.G.B.; Pippi, B.; Koester, L.S.; Fuentefria, A.M.; Limberger, R.P. A chitosanhydrogel-thickened nanoemulsion containing Pelargonium graveolens essential oil for treatment of vaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 56, 101527. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; de Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.M.; Aboelenin, S.M.; Alfaifi, M.Y.; Shati, A.A.; Elbehairi, S.E.; Elshaarawy, R.F.M.; Abd Elwahab, N.H. Quaternized chitosan thiol hydrogel-thickened nanoemulsion: A multifunctional platform for upgrading the topical applications of virgin olive oil. Pharmaceutics 2022, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Tayalia, P. Clove oil-incorporated antibacterial gelatin–chitosan cryogels for tissue engineering: An in vitro study. ACS Biomater. Sci. Eng. 2022, 8, 3557–3567. [Google Scholar] [CrossRef]
- Mirzaei, B.A.; Ramazani, A.S.A.; Shafiee, M.; Danaei, M. Studies on Glutaraldehyde Crosslinked Chitosan Hydrogel Properties for Drug Delivery Systems. Int. J. Polym. Mater. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- Zandraa, O.; Ngwabebhoh, F.A.; Patwa, R.; Nguyen, H.T.; Motiei, M.; Saha, N.; Saha, T.; Saha, P. Development of dual crosslinked mumio-based hydrogel dressing for wound healing application: Physico-chemistry and antimicrobial activity. Int. J. Pharm. 2021, 607, 120952. [Google Scholar] [CrossRef]
- Hu, K.; Jia, E.; Zhang, Q.; Zheng, W.; Sun, R.; Qian, M.; Tan, Y.; Hu, W. Injectable carboxymethyl chitosan-genipin hydrogels encapsulating tea tree oil for wound healing. Carbohydr. Polym. 2023, 301, 120348. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Zandraa, O.; Patwa, R.; Saha, N.; Capakova, Z.; Saha, P. Self-crosslinked chitosan/dialdehyde xanthan gum blended hypromellose hydrogel for the controlled delivery of ampicillin, minocycline and rifampicin. Int. J. Biol. Macrom. 2021, 167, 1468–1478. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S.; Arthanareeswaran, G. Biomass-derived dialdehyde cellulose cross-linked chitosan-based nanocomposite hydrogel with phytosynthesized Zinc oxide nanoparticles for enhanced curcumin delivery and bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- De Nooy, A.E.J.; Besemer, A.C.; Van Bekkum, H.; Van Dijk, J.A.P.P.; Smit, J.A.M. TEMPO-mediated oxidation of pullulan and influence of ionic strength and linear charge density on the dimensions of the obtained polyelectrolyte chains. Macromolecules 1996, 29, 6541–6547. [Google Scholar] [CrossRef]
- Brunnel, D.; Shacht, E. Chemical modification of pullulan. 1. Periodate oxidation. Polymer 1993, 34, 2628–2632. [Google Scholar] [CrossRef]
- Constantin, M.; Spiridon, M.; Ichim, D.L.; Daraba, O.M.; Suflet, D.M.; Ignat, M.; Fundueanu, G. Synthesis, biological and catalytic activity of silver nanoparticles generated and covered by oxidized pullulan. Mat. Chem. Phys. 2023, 295, 127141. [Google Scholar] [CrossRef]
- Baron, R.I.; Duceac, I.A.; Morariu, S.; Bostanaru-Iliescu, A.-C.; Coseri, S. Hemostatic cryogels based on oxidized pullulan/dopamine with potential use as wound dressings. Gels 2022, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, W.; Liu, Y.; Li, W.; Fan, D.; Zhu, C.; Wang, Y. HLC/pullulan and pullulan hydrogels: Their microstructure, engineering process and biocompatibility. Mater. Sci. Eng. C 2016, 58, 1046–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Qian, Y.; Zhang, Z.; Lyu, L.; Wang, Y.; Ye, F. Study on the electrospinning of gelatin/pullulan composite nanofibers. Polymers 2019, 11, 1424. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Helyon 2024, 10, e22437. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Rasul, A.; Waqas, M.K.; Saadullah, M.; Aslam, N.; Abbas, G.; Latif, S.; Afzal, H.; Inam, S.; Akhtar Shah, P. Formulation and Evaluation of a Clove Oil-Encapsulated Nanofiber Formulation for Effective Wound-Healing. Molecules 2021, 26, 2491. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Cabrera, S.; Benavides, J.; Lopera, Y.; Yarce, C.J. Lipidic matrixes containing clove essential oil: Biological activity, microstructural and textural studies. Molecules 2021, 26, 2425. [Google Scholar] [CrossRef] [PubMed]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemusions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Artif. Cellsnanomed. Biotechnol. 2017, 45, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Khunkitti, W.; Veerapan, P.; Hahnvajanawong, C. In vitro bioactivities of clove buds oil (Eugenia caryophyllata) and its effect on dermal fibroblast. Int. J. Pharm. Pharm. Sci. 2012, 4, 556–560. [Google Scholar]
- Stoleru, E.; Dumitriu, R.P.; Ailiesei, G.L.; Yilmaz, C.; Brebu, M. Synthesis of bioactive materials by in situ one-step direct loading of Syzygium aromaticum essential oil into chitosan-based hydrogels. Gels 2022, 8, 225. [Google Scholar] [CrossRef]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocoll. 2012, 27, 316–323. [Google Scholar] [CrossRef]
- Purwanti, N.; Ichikawa, S.; Neves, M.A.; Uemura, K.; Nakajima, M.; Kobayashi, I. β-lactoglobulin as Food Grade Surfactant for Clove Oil-in-Water and Limonene-in-Water Emulsion Droplets Produced by Microchannel Emulsification. Food Hydrocoll. 2016, 60, 98–108. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Yang, L.; Paulson, A.T. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res. Int. 2000, 33, 571–578. [Google Scholar] [CrossRef]
- Pelin, I.M.; Popescu, I.; Calin, M.; Rebleanu, D.; Voicu, G.; Ionita, D.; Zaharia, M.M.; Constantin, M.; Fundueanu, G. Tri-component hydrogel as template for nanocrystalline hydoxyapatite deposition using alternate soaking method for bone tissue engineering applications. Gels 2023, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Duceac, I.A.; Coseri, S. Chitosan Schiff-base hydrogels-A critical perspective review. Gels 2022, 28, 779. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; David, G.; Serbezeanu, D.; Rimbu, C.M.; Daraba, O.M.; Enache, A.A.; Bercea, M. Phosphorylated curdlan gel/polyvinyl alcohol electrospun nanofibres loaded with clove oil with antibacterial activity. Gels 2022, 8, 439. [Google Scholar] [CrossRef]
- Tarhan, I.; Bakir, M.R.; Kalkan, O.; Yontem, M.; Kara, H. Rapid determination of adulteration of clove essential oil with benzyl alcohol and ethyl acetate: Towards quality control analysis by FTIR with chemometrics. Vib. Spectrosc. 2022, 118, 103339. [Google Scholar] [CrossRef]
- Wang, L.-H.; Sung, W.-C. Rapid evaluation and quantitative analysis of eugenol derivatives in essential oils and cosmetic formulations on human skin using attenuated total reflectance–infrared spectroscopy. J. Spectrosc. 2011, 26, 176163. [Google Scholar] [CrossRef]
- Taraj, K.; Andoni, A.; Ylli, F.; Ylli, A.; Hoxha, R.; Llupa, J.; Malollari, I. Spectroscopic investigation of Syzygium aromaticum L. oil by water distillation extraction. J. Int. Environ. Appl. Sci. 2019, 15, 122–126. [Google Scholar]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effects of the type of solvent acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; Luchese, C.L.; Tessaro, I.C. Impact of acid type for chitosan dissolution on the characteristics and biodegradability of cornstarch/chitosan based films. Macromolecules 2019, 138, 693–703. [Google Scholar] [CrossRef]
- Păucean, A.; Vodnar, D.C.; Mureșan, V.; Fetea, F.; Ranga, F.; Man, S.M.; Muste, S.; Socaciu, C. Monitoring lactic acid concentrations by infrared spectroscopy: A new developed method for Lactobacillus fermenting media with potential food applications. Acta Aliment. 2017, 46, 420–427. [Google Scholar] [CrossRef]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidized dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mat. Sci. Mat. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef]
- Niamsa, N.; Baimark, Y. Preparation and characterization of highly flexible chitosan films for use as food packaging. Am. J. Food Technol. 2009, 4, 162–169. [Google Scholar] [CrossRef]
- Bölgen, N.; Demir, D.; Yalçin, M.S.; Özdemir, S. Development of Hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1581–1590. [Google Scholar] [CrossRef]
- Gottrup, F. Oxygen therapies for wound healing: EWMA findings and recommendations. Wounds Int. 2017, 8, 18–22. [Google Scholar]
- Avalle, M.; Belingardi, G.; Montanini, R. Characterization of polymeric structural foams under compressive impact loading by means of energy-absorption diagram. Int. J. Impact Eng. 2001, 25, 455–472. [Google Scholar] [CrossRef]
- Di Muzio, L.; Sergi, C.; Carriero, V.C.; Tirillo, J.; Adrover, A.; Messina, E.; Gaetani, R.; Petralito, S.; Casadei, M.A.; Paolicelli, P. Gelatin-based spongy and compressive resistant cryogels with shape recovery ability as ideal scaffolds to support cell adhesion for tissue regeneration. React. Funct. Polym. 2023, 189, 105607. [Google Scholar] [CrossRef]
- Yetiskin, B.; Okay, O. Silk fibroin cryogel building adaptive organohydrogels with switching mechanics and viscoelasticity. ACS Appl. Polym. Mater. 2022, 4, 5234–5245. [Google Scholar] [CrossRef]
- Ma, X.M.; Li, R.; Ren, J.; Lv, X.C.; Zhao, X.H.; Ji, Q.; Xi, Y.Z. Restorable, high-strength poly(Nisopropylacrylamide) hydrogels constructed through chitosan-based dual macro-cross-linkers with rapid response to temperature jumps. RSC Adv. 2017, 7, 47767–47774. [Google Scholar] [CrossRef]
- Du, H.; Ji, Q.; Xing, Y.; Ma, X.; Xia, Y. A general route to strong, conductive and antibacterial curdlan-based purely natural eutectohydrogels with self-assembled layer-by-layer network structure. Carbohydr. Polym. 2023, 316, 121035. [Google Scholar] [CrossRef]
- Tanpichai, S.; Oksman, K. Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and PVA: Mechanical properties and creep recovery. Compos. Part A 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlean, N. Physically cross-linked chitosan cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Macromolecules 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Seyedjafari, E.; Ardeshirylajimi, A. PCL/chitosan/Zn-doped nHA electrospun nanocomposite scaffold promotes adipose derived stem cells adhesion and proliferation. Carbohydr. Polym. 2015, 118, 133–142. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Biological and Nonbiological Antioxidant Activity of Some Essential Oils. J. Agric. Food Chem. 2016, 64, 4716–4724. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Akturk, A. Enrichment of Cellulose Acetate Nanofibrous Scaffolds with Retinyl Palmitate and Clove Essential Oil for Wound Healing Applications. ACS Omega 2023, 8, 5553–5560. [Google Scholar] [CrossRef]
- Nzeako, B.; Al-Bushra, L. Comparative studies of antimycotic potential of thyme and clove oil extracts with antifungal antibiotics on Candida albicans. Afr. J. Biotechnol. 2008, 7, 1612–1619. [Google Scholar] [CrossRef]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rimbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/poly(vinyl alcohol) hydrogels loaded with Calendula officinalis extract: Design and in vitro evaluation for wound healing applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef]
- Mostaghimi, M.; Majdinasab, M.; Hosseini, S.M.H. Characterization of Alginate hydrogel beads loaded with Thyme oil and Clove Essential oil nanoemulsions. J. Polym. Environ. 2022, 30, 1647–1661. [Google Scholar] [CrossRef]
- Chee, H.Y.; Lee, M.H. Antifungal activity of clove essential oil and its volatile vapour against dermatophytic fungi. Microbiology 2007, 35, 241–243. [Google Scholar] [CrossRef]
- Ali, B.A.; Ibrahim, O.M.S. Antifungal activity of clove (Syzygium aromaticum) essential oil extract against induced topical skin infection by Candida albicans in mice in vivo. Egypt. J. Hosp. Med. 2023, 91, 3855–3861. [Google Scholar] [CrossRef]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the antifungal activity and mode of action of Thymus vularis, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus essential oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef]
- Labib, G.S.; Aldawsari, H. Innovation of natural essential oil-loaded Orabase for local treatment of oral candidiasis. Drug Des. Devel. Ther. 2015, 9, 3349–3359. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Gamzazade, A.I.; Slimak, V.M.; Skljar, A.M.; Stykova, E.V.; Pavlova, S.S.A.; Rogozin, S.V. Investigation of the hydrodynamic properties of chitosan solutions. Acta Polym. 1985, 36, 420–424. [Google Scholar] [CrossRef]
- Zhao, H.; Heindel, N.D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Leane, M.M.; Nankervis, R.; Smith, A.; Illum, L. Use of ninhydrin assay to measure the release of chitosan from oral solid dosage forms. Int. J. Pharm. 2004, 271, 241–249. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, X.; Zhang, J.; Chen, W.; Li, X.; Wei, X.; Li, P.A. Porous Hydrogel with High Mechanical Strength and Biocompatibility for Bone Tissue Engineering. J. Funct. Biomater. 2022, 13, 140. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual Cross-Linked Chitosan/PVA Hydrogels Containing Silver Nanoparticles with Antimicrobial Properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Gulcin, I.; Elmastas, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Lambert, R.J.W.; Pearson, J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J. Appl. Microbiol. 2000, 88, 784–790. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic sensitivity testing by a standardized single disk method. Am. J. Clin. Path. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility of Infrequently Isolated or Fastidious Bacteria, Approved Guideline, 2nd ed.; CLSI document M45-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]










| Sample Code | CS Conc. (%, w/w) | CO Conc. (%, w/w) | Size (nm)/Storage Time (Hours) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | 24 h | |||
| CS/CO 1 | 1.75 | 1 | 906 ± 13 | 1163 ± 27 | 1277 ± 27 | 1430 ± 26 | 1408 ± 31 | 1636 ± 20 |
| CS/CO 2 | 2 | 1149 ± 24 | 1196 ± 47 | 1410 ± 26 | 1436 ± 14 | 1565 ± 35 | 1822 ± 31 | |
| CS/CO 3 | 2.5 | 1165 ± 17 | 1305 ± 16 | 1473 ± 28 | 1589 ± 19 | 1920 ± 36 | 2235 ± 25 | |
| CS/CO 4 | 2 | 5 | 2205 ± 25 | 2372 ± 16 | 2455 ± 26 | 2690 ± 18 | 2981 ± 72 | 3157 ± 35 |
| Sample Code | Initial Mixture | Final Composition | |||||
|---|---|---|---|---|---|---|---|
| CS/CO Emulsion Type | Molar Ratio NH2/CHO | WCS/WOP/WCO (%, w/w) | GF (%) | C.D. (%) | LC (%, w/w) | Efficiency (%) | |
| CS/OP0.5-C1 | CS/CO 2 | 1/0.5 | 61.7/19.2/19.1 | 67.6 ± 1.4 | 5.3 ± 0.8 | 12.8 ± 0.7 | 66.9 ± 6.0 |
| CS/OP1-C1 | 1/1 | 51.8/32.1/16.1 | 75.5 ± 1.8 | 11.4 ± 1.6 | 14.1 ± 1.2 | 87.8 ± 7.5 | |
| CS/OP2-C1 | 1/2 | 39.2/48.6/12.2 | 63.9 ± 1.1 | 35.9 ± 2.5 | 8.7 ± 0.4 | 71.7 ± 2.1 | |
| CS/OP1-C5 | CS/CO 4 | 1/1 | 31.5/19.6/48.9 | 53.1 ± 2.4 | 7.6 ± 0.5 | 46.5 ± 1.7 | 95.2 ± 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suflet, D.M.; Constantin, M.; Pelin, I.M.; Popescu, I.; Rimbu, C.M.; Horhogea, C.E.; Fundueanu, G. Chitosan–Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties. Gels 2024, 10, 227. https://doi.org/10.3390/gels10040227
Suflet DM, Constantin M, Pelin IM, Popescu I, Rimbu CM, Horhogea CE, Fundueanu G. Chitosan–Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties. Gels. 2024; 10(4):227. https://doi.org/10.3390/gels10040227
Chicago/Turabian StyleSuflet, Dana Mihaela, Marieta Constantin, Irina Mihaela Pelin, Irina Popescu, Cristina M. Rimbu, Cristina Elena Horhogea, and Gheorghe Fundueanu. 2024. "Chitosan–Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties" Gels 10, no. 4: 227. https://doi.org/10.3390/gels10040227
APA StyleSuflet, D. M., Constantin, M., Pelin, I. M., Popescu, I., Rimbu, C. M., Horhogea, C. E., & Fundueanu, G. (2024). Chitosan–Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties. Gels, 10(4), 227. https://doi.org/10.3390/gels10040227