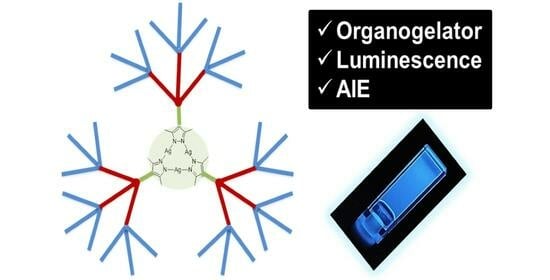
Silver Dendritic Gels with Luminescence and Aggregation-Induced Emission Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Silver Metallodendrimers
2.2. Study of Gels
2.2.1. Gel Formation
2.2.2. NMR Study
2.2.3. Luminescence and AIE Properties
3. Conclusions
4. Materials and Methods
4.1. Experimental Techniques for the Synthesis and Characterization of the Novel Compounds
4.2. Synthesis and Characterization of the Silver Dendritic Complexes
- [Ag(µ-(4-3,4,5)-10G2-APz)]3: White solid. Yield: 87%. 1H-NMR (400 MHz, C2D2Cl4, 60 °C) δ (ppm): 0.90–0.94 (m, 27H, CH3(CH2)7), 1.32–1.52 (m, 126H, CH3(CH2)7), 1.80–1.84 (m, 18H, CH2CH2OAr), 2.37 (s, 18H, CH3Pz), 3.95–4.02 (m, 18H, CH2CH2OAr), 5.04 (s, 6H, ArCH2OAr), 5.10 (s, 12H, ArCH2OAr), 6.81–6.84 (m, 6H, AA’XX’), 6.94–6.96 (m, 12H, AA’XX’), 7.19 (s, 6H, Ar-H), 7.30–7.33 (m, 6H, AA’XX’), 7.34–7.38 (m, 18H, Ar-H), 7.64–7.67 (m, 6H, AA’XX’), 7.71 (s, 3H, ArCONH2). 13C-NMR: (100 MHz, C2D2Cl4, 60 °C) δ (ppm): 13.7, 14.2, 22.7–31.9, 68.3, 68.4, 71.7, 75.0, 107.6, 114.5, 114.9, 117.3, 120.7, 128.6, 129.4, 129.6, 129.8, 130.3, 130.6, 131.2, 135.6, 142.2, 147.1, 153.1, 159.2, 159.3, 165.5. MS (MALDI+, DCTB) m/z: 3663.3 [M + Ag]+. Elemental analysis: calcd for (%) C207H282Ag3N9O21: C 69.91, H 7.99, N 3.54; found: C 70.16, H 8.21, N 3.49.
- [Ag(µ-(4-3,4,5)-12G2-APz)]3: White solid. Yield: 89%. 1H-NMR (400 MHz, C2D2Cl4, 95 °C) δ (ppm): 0.94–0.97 (m, 27H, CH3(CH2)9), 1.35–1.54 (m, 162H, CH3(CH2)9), 1.80–1.88 (m, 18H, CH2CH2OAr), 2.37 (s, 18H, CH3Pz), 3.99–4.06 (m, 18H, CH2CH2OAr), 5.10 (s, 6H, ArCH2OAr), 5.14 (s, 12H, ArCH2OAr), 6.85–6.87 (m, 6H, AA’XX’), 6.96–6.98 (m, 12H, AA’XX’), 7.22 (s, 6H, Ar-H), 7.33–7.40 (m, 24H, Ar-H), 7.61 (s, 3H, ArCONH2), 7.66–7.68 (m, 6H, AA’XX’). 13C-NMR: (100 MHz, C2D2Cl4, 95 °C) δ (ppm): 13.5, 14.0, 22.5–31.7, 68.1, 68.2, 71.5, 74.8, 107.4, 114.3, 114.6, 117.0, 120.5, 128.4, 129.1, 129.4, 129.6, 130.0, 130.7, 135.4, 142.0, 145.0, 152.9, 159.0, 159.1, 165.3. MS (MALDI+, DCTB) m/z: 3920.3 [M + Ag]+. Elemental analysis: calcd for (%) C225H318Ag3N9O21: C 70.96, H 8.42, N 3.31; found: C 70.68, H 8.75, N 3.52.
- [Ag(µ-(3,4,5-3,4,5)-10G2-APz)]3: White solid. Yield: 79%. 1H-NMR (400 MHz, CD2Cl2) δ (ppm): 0.86–0.90 (m, 81H, CH3(CH2)7), 1.27–1.47 (m, 378H, CH3(CH2)7), 1.66–1.76 (m, 54H, CH2CH2OAr), 2.36 (s, 18H, CH3Pz), 3.74 (t, 12H, J = 6.6 Hz, CH2CH2OAr), 3.84–3.91 (m, 42H, CH2CH2OAr), 5.01 (s, 6H, ArCH2OAr), 5.03 (s, 12H, ArCH2OAr), 6.60 (s, 6H, Ar-H), 6.63 (s, 12H, Ar-H), 7.23 (s, 6H, Ar-H), 7.27–7.30 (m, 6H, AA’XX’), 7.61–7.64 (m, 6H, AA’XX’), 7.88 (s, 3H, ArCONH2). 13C-NMR (100 MHz, CD2Cl2) δ (ppm): 14.3, 14.5, 23.3–32.5, 69.4, 69.5, 72.4, 73.8, 73.9, 75.7, 106.2, 106.4, 107.8, 118.4, 121.0, 130.1, 131.1, 132.1, 132.3, 133.1, 136.2, 138.2, 138.3, 142.0, 147.7, 153.5, 153.6, 153.9, 165.7. MS (MALDI+, DCTB) m/z: 6392.1 [M + Na]+, 6476.2 [M + Ag]+. Elemental analysis: calcd for (%) C387H642Ag3N9O39: C 72.98, H 10.16, N 1.96; found: C 72.61, H 10.23, N 2.12.
- [Ag(µ-(3,4,5-3,4,5)-12G2-APz)]3: White solid. Yield: 93%. 1H-NMR (400 MHz, CD2Cl2) δ (ppm): 0.87–0.90 (m, 81H, CH3(CH2)9), 1.26–1.47 (m, 486H, CH3(CH2)9), 1.70–1.75 (m, 54H, CH2CH2OAr), 2.36 (s, 18H, CH3Pz), 3.73 (t, 12H, J = 6.6 Hz, CH2CH2OAr), 3.84–3.91 (m, 42H, CH2CH2OAr), 5.00 (s, 18H, ArCH2OAr), 6.60 (s, 6H, Ar-H), 6.62 (s, 12H, Ar-H), 7.24–7.27 (m, 12H, Ar-H), 7.61-7.63 (m, 6H, AA’XX’), 7.94 (s, 3H, ArCONH2). 13C-NMR (100 MHz, CD2Cl2) δ (ppm): 14.3, 14.5, 23.3-32.5, 69.4, 69.6, 72.3, 73.8, 73.9, 75.7, 106.2, 106.4, 107.8, 116.6, 121.1, 130.0, 130.1, 131.1, 132.3, 133.1, 136.2, 138.2, 138.3, 142.0, 149.3, 153.5, 153.6, 153.9, 166.1. MS (MALDI+, DCTB) m/z: 7146.2 [M + Na]+. Elemental analysis: calcd for (%) C441H750Ag3N9O39: C 74.33, H 10.61, N 1.77; found: C 74.61, H 10.69, N 1.70.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Grover, G.; Weiss, R.G. Luminescent Behavior of Gels and Sols Comprised of Molecular Gelators. Gels 2021, 7, 19. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent gels: A review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Galindo, J.M.; Tardío, C.; Saikia, B.; Van Cleuvenbergen, S.; Torres-Moya, I. Recent Insights about the Role of Gels in Organic Photonics and Electronics. Gels 2023, 9, 875. [Google Scholar] [CrossRef]
- Tavakoli, J.; Ghahfarokhi, A.J.; Tang, Y. Aggregation-Induced Emission Fluorescent Gels: Current Trends and Future Perspectives. Top. Curr. Chem. 2021, 379, 9. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.T.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. A Photostable AIE Luminogen for Specific Mitochondrial Imaging and Tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Xie, H.; Ma, Y.; Liu, C.; Liu, S.; Liu, M. Emissive intelligent supramolecular gel for highly selective sensing of Al3+ and writable soft material. Chem. Commun. 2018, 54, 13674–13677. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.M.; Fan, Q.H. Supramolecular organogels based on dendrons and dendrimers. Chem. Asian J. 2014, 9, 1724–1750. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.-D.; Jiang, D.-L.; Aida, T. Dendritic Physical Gel: Hierarchical Self-Organization of a Peptide-Core Dendrimer to Form a Micrometer-Scale Fibrous Assembly. J. Am. Chem. Soc. 2000, 122, 3232–3233. [Google Scholar] [CrossRef]
- Newkome, G.R.; Baker, G.R.; Saunders, M.J.; Russo, P.S.; Gupta, V.K.; Yao, Z.-q.; Miller, J.E.; Bouillion, K. Two-directional cascade molecules: Synthesis and characterization of [9]-n-[9] arborols. J. Chem. Soc. Chem. Commun. 1986, 752–753. [Google Scholar] [CrossRef]
- Smith, D.K. Dendritic gels—Many arms make light work. Adv. Mater. 2006, 18, 2773–2778. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Z.-T.; Liu, J.; He, Y.-M.; Zheng, Q.-Y.; Fan, Q.-H. Peripherally Dimethyl Isophthalate-Functionalized Poly(benzyl ether) Dendrons: A New Kind of Unprecedented Highly Efficient Organogelators. J. Am. Chem. Soc. 2009, 131, 7950–7951. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Z.; Chen, H.; Fan, Q.-H. Functional supramolecular gels based on poly(benzyl ether) dendrons and dendrimers. Chem. Commun. 2022, 58, 8736–8753. [Google Scholar] [CrossRef]
- Iguarbe, V.; Romero, P.; Barberá, J.; Elduque, A.; Giménez, R. Dual liquid Crystalline/Gel behavior with AIE effect promoted by Self-assembly of pyrazole dendrons. J. Mol. Liq. 2022, 365, 120109. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Zhang, G.; Yang, X.; Feng, Y.; Fan, Q.; Zhu, D. Multicolor Tunable Emission from Organogels Containing Tetraphenylethene, Perylenediimide, and Spiropyran Derivatives. Adv. Funct. Mater. 2010, 20, 3244–3251. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lv, Y.X.; Han, Y.; Zhu, B.; Zhang, F.; Bo, Z.S.; Liu, C.Y. Dendritic Effect on Supramolecular Self-Assembly: Organogels with Strong Fluorescence Emission Induced by Aggregation. Langmuir 2009, 25, 8548–8555. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Serrano, J.L.; Sierra, T.; Ballesteros, A.; de Saa, D.; Barluenga, J. Control of self-assembly of a 3-hexen-1,5-diyne derivative: Toward soft materials with an aggregation-induced enhancement in emission. J. Am. Chem. Soc. 2011, 133, 8110–8113. [Google Scholar] [CrossRef]
- Rajamalli, P.; Prasad, E. Low Molecular Weight Fluorescent Organogel for Fluoride Ion Detection. Org. Lett. 2011, 13, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Rajamalli, P.; Prasad, E. Non-amphiphilic pyrene cored poly(aryl ether) dendron based gels: Tunable morphology, unusual solvent effects on the emission and fluoride ion detection by the self-assembled superstructures. Soft Matter 2012, 8, 8896–8903. [Google Scholar] [CrossRef]
- Rajamalli, P.; Prasad, E. Tunable Morphology and Mesophase Formation by Naphthalene-Containing Poly(aryl ether) Dendron-Based Low-Molecular-Weight Fluorescent Gels. Langmuir 2013, 29, 1609–1617. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Deng, G.-J.; Liu, Z.-X.; He, Y.-M.; Fan, Q.-H. Fluorescent Dendritic Organogels Based on 2-(2′-Hydroxyphenyl)benzoxazole: Emission Enhancement and Multiple Stimuli-Responsive Properties. Chem. Eur. J. 2015, 21, 11018–11028. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from Coordination Complexes, Organometallic, and Coordination Polymers. Chem. Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef] [PubMed]
- Fages, F. Metal Coordination To Assist Molecular Gelation. Angew. Chem. Int. Ed. 2006, 45, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Coord. Chem. Rev. 2013, 257, 1373–1408. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjøniksen, A.-L.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, Applicability, and Advanceability. Adv. Mater. 2019, 31, 1806204. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Chu, Q.; Feng, Y. Recent Advances in Stimuli-Responsive Metallogels. Molecules 2023, 28, 2274. [Google Scholar] [CrossRef]
- Shao, T.; Falcone, N.; Kraatz, H.-B. Supramolecular Peptide Gels: Influencing Properties by Metal Ion Coordination and Their Wide-Ranging Applications. ACS Omega 2020, 5, 1312–1317. [Google Scholar] [CrossRef]
- Fu, H.L.K.; Yam, V.W.W. Highlight review supramolecular metallogels of platinum(II) and gold(III) complexes. Chem. Lett. 2018, 47, 605–610. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Feng, Y.; Zhao, Z.-Y.; Yan, Z.-C.; He, Y.-M.; Luo, X.-J.; Liu, C.-Y.; Fan, Q.-H. A New Class of Dendritic Metallogels with Multiple Stimuli-Responsiveness and as Templates for the In Situ Synthesis of Silver Nanoparticles. Chem. Eur. J. 2014, 20, 533–541. [Google Scholar] [CrossRef]
- Xue, M.; Lü, Y.; Sun, Q.; Liu, K.; Liu, Z.; Sun, P. Ag(I)-Coordinated Supramolecular Metallogels Based on Schiff Base Ligands: Structural Characterization and Reversible Thixotropic Property. Cryst. Growth Des. 2015, 15, 5360–5367. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Li, W.; Wu, L. Structural Characterization and Chemical Response of a Ag-Coordinated Supramolecular Gel. Langmuir 2007, 23, 8217–8223. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H.; Lee, M. Stimuli-Responsive Gels from Reversible Coordination Polymers. Angew. Chem. Int. Ed. 2005, 44, 5810–5814. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.-H.; Li, D. Coinage-Metal-Based Cyclic Trinuclear Complexes with Metal–Metal Interactions: Theories to Experiments and Structures to Functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef]
- Kishimura, A.; Yamashita, T.; Aida, T. Phosphorescent Organogels via “Metallophilic” Interactions for Reversible RGB−Color Switching. J. Am. Chem. Soc. 2005, 127, 179–183. [Google Scholar] [CrossRef]
- Cored, J.; Crespo, O.; Serrano, J.L.; Elduque, A.; Giménez, R. Decisive Influence of the Metal in Multifunctional Gold, Silver, and Copper Metallacycles: High Quantum Yield Phosphorescence, Color Switching, and Liquid Crystalline Behavior. Inorg. Chem. 2018, 57, 12632–12640. [Google Scholar] [CrossRef]
- Iguarbe Montalbán, V. Dendrones y Metalodendrímeros para Materiales Blandos: Estudio de la Autoorganización en Cristales Líquidos y Geles. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2020. [Google Scholar]
- Balagurusamy, V.S.K.; Ungar, G.; Percec, V.; Johansson, G. Rational Design of the First Spherical Supramolecular Dendrimers Self-Organized in a Novel Thermotropic Cubic Liquid-Crystalline Phase and the Determination of Their Shape by X-ray Analysis. J. Am. Chem. Soc. 1997, 119, 1539–1555. [Google Scholar] [CrossRef]
- Moyano, S.; Barberá, J.; Diosdado, B.E.; Serrano, J.L.; Elduque, A.; Giménez, R. Self-assembly of 4-aryl-1H-pyrazoles as a novel platform for luminescent supramolecular columnar liquid crystals. J. Mater. Chem. C 2013, 1, 3119–3128. [Google Scholar] [CrossRef]








| Compound | Ethanol | Ethyl Acetate | Dichloromethane | Cyclohexane | Dodecane |
|---|---|---|---|---|---|
| [Ag(µ-(4-3,4,5)-10G2-APz)]3 | I | I | I | I | G [1.3] |
| [Ag(µ-(4-3,4,5)-12G2-APz)]3 | I | I | I | I | G [1.1] |
| [Ag(µ-(3,4,5-3,4,5)-10G2-APz)]3 | I | I | S | G [2.2] | P |
| [Ag(µ-(3,4,5-3,4,5)-12G2-APz)]3 | I | I | S | P | P |
| Compound | 5 wt% | 4 wt% | 3 wt% | 2 wt% |
|---|---|---|---|---|
| [Ag(µ-(4-3,4,5)-10G2-APz)]3 | 81 a | 75 a | 66 a | 56 a |
| [Ag(µ-(4-3,4,5)-12G2-APz)]3 | 86 a | 78 a | 70 a | 64 a |
| [Ag(µ-(3,4,5-3,4,5)-10G2-APz)]3 | 54 b | 41 b | 35 b | - |
| Compound | λgel a (nm) | λsol b (nm) | λTHFdil a,c (nm) | λTHFconc a,d (nm) | AIE |
|---|---|---|---|---|---|
| [Ag(µ-(4-3,4,5)-10G2-APz)]3 | 423 e,g | 418 e,g | 356 | 440 g | 61 |
| [Ag(µ-(4-3,4,5)-12G2-APz)]3 | 425 e,g | 419 e,g | 346 | 445 g | 58 |
| [Ag(µ-(3,4,5-3,4,5)-10G2-APz)]3 | 449 f,h | 439 f,h | 355 | 453 h | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iguarbe, V.; Romero, P.; Elduque, A.; Giménez, R. Silver Dendritic Gels with Luminescence and Aggregation-Induced Emission Effect. Gels 2024, 10, 291. https://doi.org/10.3390/gels10050291
Iguarbe V, Romero P, Elduque A, Giménez R. Silver Dendritic Gels with Luminescence and Aggregation-Induced Emission Effect. Gels. 2024; 10(5):291. https://doi.org/10.3390/gels10050291
Chicago/Turabian StyleIguarbe, Verónica, Pilar Romero, Anabel Elduque, and Raquel Giménez. 2024. "Silver Dendritic Gels with Luminescence and Aggregation-Induced Emission Effect" Gels 10, no. 5: 291. https://doi.org/10.3390/gels10050291
APA StyleIguarbe, V., Romero, P., Elduque, A., & Giménez, R. (2024). Silver Dendritic Gels with Luminescence and Aggregation-Induced Emission Effect. Gels, 10(5), 291. https://doi.org/10.3390/gels10050291