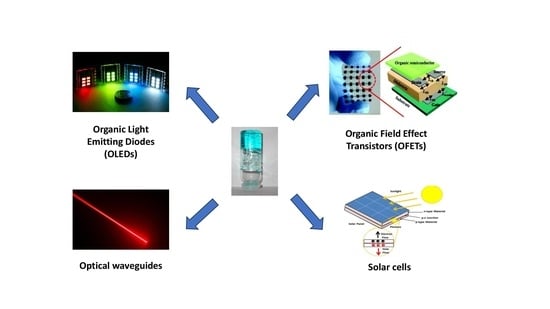
Recent Insights about the Role of Gels in Organic Photonics and Electronics
Abstract
:1. Introduction
2. Gels in Organic Field-Effect Transistors (OFETs)
3. Gels in Solar Cells
4. Gels in Organic Light-Emitting Diodes (OLEDs)
5. Gels in Optical Waveguides

6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lloyd, D.J.; Alexander, J. Colloid Chemistry; Chemical Catalogue Company: New York, NY, USA, 1926. [Google Scholar]
- Graham, T. Liquid diffusion applied to analysis. Philos. Trans. Royal. Soc. Lond. 1861, 151, 183–224. [Google Scholar] [CrossRef]
- Sheng, F.; Zhang, B.; Zhang, Y.; Li, Y.; Cheng, R.; Wei, C.; Ning, C.; Dong, K.; Wang, Z.L. Ultrastretchable Organogel/Silicone Fiber-Helical Sensors for Self-Powered Implantable Ligament Strain Monitoring. ACS Nano 2022, 16, 10958–10967. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Shan, J.; Guo, C.; Wang, Y. Selective Colorimetric and Fluorometric Organogel Sensors for the Detection of F− and ClO− Based on Chiral Glutamic and Phenothiazine Derivatives. Colloid Polym. Sci. 2023, 301, 107–115. [Google Scholar] [CrossRef]
- Ge, J.; Dai, S.; Dong, X.; Li, M.; Xu, Y.; Jiang, Y.; Yuan, N.; Ding, J. A Wide-Temperature-Range Sensor Based on Wide-Strain-Range Self-Healing and Adhesive Organogels. New J. Chem. 2022, 46, 4334–4342. [Google Scholar] [CrossRef]
- Koo, J.; Lim, S.-I.; Jang, J.; Oh, M.; Jeong, K.-U. From Polymer Gels to 3D Actuators: Transformation of Programmed 2D Structures to 3D Objects. J. Chem. Educ. 2020, 97, 1396–1401. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Li, Y. Recent Advances in Plasticized PVC Gels for Soft Actuators and Devices: A Review. J. Mater. Chem. C Mater. Opt. Electron. Devices 2019, 7, 12991–13009. [Google Scholar] [CrossRef]
- Hwang, T.; Frank, Z.; Neubauer, J.; Kim, K.J. High-Performance Polyvinyl Chloride Gel Artificial Muscle Actuator with Graphene Oxide and Plasticizer. Sci. Rep. 2019, 9, 9658. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chakraborty, E. Hydrogel Based Tissue Engineering and Its Future Applications in Personalized Disease Modeling and Regenerative Therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, Promising Drug Delivery Systems: An Update of State-of-the-Art and Recent Applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Mashabela, L.T.; Maboa, M.M.; Miya, N.F.; Ajayi, T.O.; Chasara, R.S.; Milne, M.; Mokhele, S.; Demana, P.H.; Witika, B.A.; Siwe-Noundou, X.; et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels 2022, 8, 563. [Google Scholar] [CrossRef]
- Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Damodaran, K.K.; Maurin, A.; Day, G.M.; Thompson, H.P.G.; Cameron, G.J.; Bernal, J.C.; Steed, J.W. Pharmaceutical Polymorph Control in a Drug-Mimetic Supramolecular Gel. Chem. Sci. 2017, 8, 78–84. [Google Scholar] [CrossRef]
- Aparicio, F.; Matesanz, E.; Sánchez, L. Cooperative Self-Assembly of Linear Organogelators. Amplification of Chirality and Crystal Growth of Pharmaceutical Ingredients. Chem. Commun. 2012, 48, 5757. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moya, I.; Sánchez, A.; Saikia, B.; Yufit, D.S.; Prieto, P.; Carrillo, J.R.; Steed, J.W. Highly Thermally Resistant Bisamide Gelators as Pharmaceutical Crystallization Media. Gels 2023, 9, 26. [Google Scholar] [CrossRef]
- Kaliaraj, G.; Shanmugam, D.; Dasan, A.; Mosas, K. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent Advances in 3D Printing of Tough Hydrogels: A Review. Compos. B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Silva, P.M.; Martins, A.J.; Fasolin, L.H.; Vicente, A.A. Modulation and Characterization of Wax-Based Olive Oil Organogels in View of Their Application in the Food Industry. Gels 2021, 7, 12. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Garti, N. An Overview of the Past, Present, and Future of Organogels. In Edible Oleogels; Marangoni, A.G., Garti, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–17. ISBN 9780983079118. [Google Scholar]
- Mehta, C.; Bhatt, G.; Kothiyal, P. A Review on Organogel for Skin Aging. Indian J. Pharm. Biol. Res. 2016, 4, 28–37. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Nandi, A.K.; Chatterjee, D.P. Hybrid Polymer Gels for Energy Applications. J. Mater. Chem. A Mater. Energy Sustain. 2023, 11, 12593–12642. [Google Scholar] [CrossRef]
- Lal, J.; Biswas, P.; Singh, S.K.; Debbarma, R.; Mehta, N.K.; Deb, S.; Sharma, S.; Waikhom, G.; Patel, A.B. Moving towards Gel for Fish Feeding: Focus on Functional Properties and Its Acceptance. Gels 2023, 9, 305. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Tajarudin, H.A.; Balakrishnan, V.; Nasution, H. Insights into the Role of Biopolymer-Based Xerogels in Biomedical Applications. Gels 2022, 8, 334. [Google Scholar] [CrossRef]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main Features and Applications of Organogels in Cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef]
- Esposito, C.L.; Kirilov, P. Preparation, Characterization and Evaluation of Organogel-Based Lipstick Formulations: Application in Cosmetics. Gels 2021, 7, 97. [Google Scholar] [CrossRef]
- Mosquera Narvaez, L.E.; Ferreira, L.M. de M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. [Google Scholar] [CrossRef]
- Babu, S.S.; Prasanthkumar, S.; Ajayaghosh, A. Self-Assembled Gelators for Organic Electronics. Angew. Chem. Int. Ed. Engl. 2012, 51, 1766–1776. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-P.; Um, M.-C.; Nam, S.-R.; Hong, J.-I.; Lee, S. Organic Single-Nanofiber Transistors from Organogels. Chem. Commun. 2009, 3, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-W.; Tevis, I.D.; Tayi, A.S.; Cui, H.; Stupp, S.I. Semiconducting Nanowires from Hairpin-Shaped Self-Assembling Sexithiophenes. J. Phys. Chem. B. 2010, 114, 14778–14786. [Google Scholar] [CrossRef]
- Guan, Y.-S.; Qin, Y.; Sun, Y.; Wang, C.; Xu, W.; Zhu, D. Single-Bundle Nanofiber Based OFETs Fabricated from a Cyclic Conjugated Organogelator with High Field-Effect Mobility and High Photoresponsivity. Chem. Commun. 2015, 51, 12182–12184. [Google Scholar] [CrossRef] [PubMed]
- Alpaslan Kösemen, Z.; Kösemen, A.; Öztürk, S.; Canımkurbey, B.; Yerlİ, Y. High Mobility and Low Operation Voltage Organic Field Effect Transistors by Using Polymer-Gel Dielectric and Molecular Doping. Mater. Sci. Semicond. Process. 2017, 66, 207–211. [Google Scholar] [CrossRef]
- Jeng, J.-Y.; Chiang, Y.-F.; Lee, M.-H.; Peng, S.-R.; Guo, T.-F.; Chen, P.; Wen, T.-C. CH3NH3PbI3 Perovskite/Fullerene Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-D.; Cui, C.; Li, Y.-Q.; Zhou, L.; Ou, Q.-D.; Li, C.; Li, Y.; Tang, J.-X. Single-Junction Polymer Solar Cells Exceeding 10% Power Conversion Efficiency. Adv. Mater. 2015, 27, 1035–1041. [Google Scholar] [CrossRef]
- Luo, D.; Jang, W.; Babu, D.D.; Kim, M.S.; Wang, D.H.; Kyaw, A.K.K. Recent Progress in Organic Solar Cells Based on Non-Fullerene Acceptors: Materials to Devices. J. Mater. Chem. A Mater. Energy Sustain. 2022, 10, 3255–3295. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-Generation Organic Photovoltaics Based on Non-Fullerene Acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Raut, P.; Kishnani, V.; Mondal, K.; Gupta, A.; Jana, S.C. A Review on Gel Polymer Electrolytes for Dye-Sensitized Solar Cells. Micromachines 2022, 13, 680. [Google Scholar] [CrossRef]
- Kang, M.-S.; Ahn, K.-S.; Lee, J.-W. Quasi-Solid-State Dye-Sensitized Solar Cells Employing Ternary Component Polymer-Gel Electrolytes. J. Power Sources 2008, 180, 896–901. [Google Scholar] [CrossRef]
- Dong, R.-X.; Shen, S.-Y.; Chen, H.-W.; Wang, C.-C.; Shih, P.-T.; Liu, C.-T.; Vittal, R.; Lin, J.-J.; Ho, K.-C. A Novel Polymer Gel Electrolyte for Highly Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. A Mater. Energy Sustain. 2013, 1, 8471. [Google Scholar] [CrossRef]
- Alinejad, M.; Buraidah, M.H.; Teo, L.P.; Arof, A.K. Saffron Dye-Sensitized Solar Cells with Polyvinyl Alcohol Based Gel Polymer Electrolytes. Opt. Quantum Electron. 2023, 55, 804. [Google Scholar] [CrossRef]
- Passantino, J.M.; Wolfe, K.D.; Simon, K.T.; Cliffel, D.E.; Jennings, G.K. Photosystem I Enhances the Efficiency of a Natural, Gel-Based Dye-Sensitized Solar Cell. ACS Appl. Bio Mater. 2020, 3, 4465–4473. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Lin, L.; Pei, F.; Xiao, M.; Yang, X.; Yuan, G.; Zhu, C.; Chen, Y.; Chen, Q. Gelation of Hole Transport Layer to Improve the Stability of Perovskite Solar Cells. Nanomicro Lett. 2023, 15, 175. [Google Scholar] [CrossRef]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, e2005630. [Google Scholar] [CrossRef] [PubMed]
- Sekine, C.; Tsubata, Y.; Yamada, T.; Kitano, M.; Doi, S. Recent Progress of High Performance Polymer OLED and OPV Materials for Organic Printed Electronics. Sci. Technol. Adv. Mater. 2014, 15, 034203. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.D.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in Conjugated Polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Lee, B.R.; Kim, J.-W.; Kang, D.; Lee, D.W.; Ko, S.-J.; Lee, H.J.; Lee, C.-L.; Kim, J.Y.; Shin, H.S.; Song, M.H. Highly Efficient Polymer Light-Emitting Diodes Using Graphene Oxide as a Hole Transport Layer. ACS Nano 2012, 6, 2984–2991. [Google Scholar] [CrossRef] [PubMed]
- Han, T.-H.; Choi, M.-R.; Jeon, C.-W.; Kim, Y.-H.; Kwon, S.-K.; Lee, T.-W. Ultrahigh-Efficiency Solution-Processed Simplified Small-Molecule Organic Light-Emitting Diodes Using Universal Host Materials. Sci. Adv. 2016, 2, e1601428. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent Gels: A Review of Synthesis, Properties, Applications and Challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Mehwish, N.; Dou, X.; Zhao, Y.; Feng, C.-L. Supramolecular Fluorescent Hydrogelators as Bio-Imaging Probes. Mater. Horiz. 2019, 6, 14–44. [Google Scholar] [CrossRef]
- Martín, C.; Kennes, K.; Van der Auweraer, M.; Hofkens, J.; de Miguel, G.; García-Frutos, E.M. Self-Assembling Azaindole Organogel for Organic Light-Emitting Devices (OLEDs). Adv. Funct. Mater. 2017, 27, 1702176. [Google Scholar] [CrossRef]
- De, J.; Gupta, S.P.; Sudheendran Swayamprabha, S.; Dubey, D.K.; Bala, I.; Sarkar, I.; Dey, G.; Jou, J.-H.; Ghosh, S.; Pal, S.K. Blue Luminescent Organic Light Emitting Diode Devices of a New Class of Star-Shaped Columnar Mesogens Exhibiting π–π Driven Supergelation. J. Phys. Chem. C Nanomater. Interfaces 2018, 122, 23659–23674. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B Mater. Biol. Med. 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef]
- Melikov, R.; Daniel Aaron Press; Kumar, B.G.; Dogru, I.B.; Sadeghi, S.; Chirea, M.; Yılgör, İ.; Nizamoglu, S. Silk-Hydrogel Lenses for Light-Emitting Diodes. Sci. Rep. 2017, 7, 7258. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Lübben, J.F. Recent Progress in Self-Healable Hydrogel-Based Electroluminescent Devices: A Comprehensive Review. Gels 2023, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, B.; Yan, D. Recent Advances on Molecular Crystalline Luminescent Materials for Optical Waveguides. Adv. Opt. Mater. 2021, 9, 2001768. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Gather, M.C.; Yun, S.H. All-biomaterial Laser Using Vitamin and Biopolymers. Adv. Mater. 2013, 25, 5943–5947. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, C.; Ke, D.; Ye, F.; Tu, J.; Wang, L.; Lu, Y. Ultrasoft and Highly Stretchable Hydrogel Optical Fibers for in Vivo Optogenetic Modulations. Adv. Opt. Mater. 2018, 6, 1800427. [Google Scholar] [CrossRef]
- Ding, B.; Zeng, P.; Huang, Z.; Dai, L.; Lan, T.; Xu, H.; Pan, Y.; Luo, Y.; Yu, Q.; Cheng, H.-M.; et al. A 2D Material–Based Transparent Hydrogel with Engineerable Interference Colours. Nat. Commun. 2022, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; Fok, S.; Lee, V.; Rye, K.-A.; Di Girolamo, N.; Cochran, B.J. Use of High-Refractive Index Hydrogels and Tissue Clearing for Large Biological Sample Imaging. Gels 2022, 8, 32. [Google Scholar] [CrossRef]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.W.; Xie, D.; Liu, Z.; Yang, J. Polymeric Biomaterials for Biophotonic Applications. Bioact. Mater. 2018, 3, 434–445. [Google Scholar] [CrossRef]
- Guo, J.; Yang, C.; Dai, Q.; Kong, L. Soft and Stretchable Polymeric Optical Waveguide-Based Sensors for Wearable and Biomedical Applications. Sensors 2019, 19, 3771. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, L.; Gong, L.; Hu, W.; Huang, W.; Chen, Y.; Asha, A.B.; Srinivas, S.; Chen, L.; Narain, R.; et al. Injectable, Self-Healing Hydrogel with Tunable Optical, Mechanical, and Antimicrobial Properties. Chem. Mater. 2019, 31, 2366–2376. [Google Scholar] [CrossRef]
- Shan, D.; Zhang, C.; Kalaba, S.; Mehta, N.; Kim, G.B.; Liu, Z.; Yang, J. Flexible Biodegradable Citrate-Based Polymeric Step-Index Optical Fiber. Biomaterials 2017, 143, 142–148. [Google Scholar] [CrossRef]
- Choi, M.; Choi, J.W.; Kim, S.; Nizamoglu, S.; Hahn, S.K.; Yun, S.H. Light-Guiding Hydrogels for Cell-Based Sensing and Optogenetic Synthesis in Vivo. Nat. Photonics 2013, 7, 987–994. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Nazempour, R.; Zhang, Q.; Fu, R.; Sheng, X. Biocompatible and Implantable Optical Fibers and Waveguides for Biomedicine. Materials 2018, 11, 1283. [Google Scholar] [CrossRef]
- Humar, M.; Kwok, S.J.J.; Choi, M.; Yetisen, A.K.; Cho, S.; Yun, S.-H. Toward Biomaterial-Based Implantable Photonic Devices. Nanophotonics 2017, 6, 414–434. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical Cellulose Fiber Made from Regenerated Cellulose and Cellulose Acetate for Water Sensor Applications. Cellulose 2020, 27, 1543–1553. [Google Scholar] [CrossRef]
- Fujiwara, E.; Cabral, T.D.; Sato, M.; Oku, H.; Cordeiro, C.M.B. Agarose-Based Structured Optical Fibre. Sci. Rep. 2020, 10, 7035. [Google Scholar] [CrossRef]
- Zhu, Z.; Sathitsuksanoh, N.; Vinzant, T.; Schell, D.J.; McMillan, J.D.; Zhang, Y.-H.P. Comparative Study of Corn Stover Pretreated by Dilute Acid and Cellulose Solvent-Based Lignocellulose Fractionation: Enzymatic Hydrolysis, Supramolecular Structure, and Substrate Accessibility. Biotechnol. Bioeng. 2009, 103, 715–724. [Google Scholar] [CrossRef]
- Gupta, R.; Goddard, N.J. A Study of Diffraction-Based Chitosan Leaky Waveguide (LW) Biosensors. Analyst 2021, 146, 4964–4971. [Google Scholar] [CrossRef]
- Prajzler, V.; Arif, S.; Min, K.; Kim, S.; Nekvindova, P. All-Polymer Silk-Fibroin Optical Planar Waveguides. Opt. Mater. 2021, 114, 110932. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Martínez-Serrano, R.D.; Cuétara-Guadarrama, F.; Vonlanthen, M.; Illescas, J.; Zhu, X.-X.; Rivera, E. Facile Obtainment of Fluorescent PEG Hydrogels Bearing Pyrene Groups by Frontal Polymerization. Polymers 2023, 15, 1687. [Google Scholar] [CrossRef]
- Liu, H.; Wei, S.; Qiu, H.; Zhan, B.; Liu, Q.; Lu, W.; Zhang, J.; Ngai, T.; Chen, T. Naphthalimide-based Aggregation-induced Emissive Polymeric Hydrogels for Fluorescent Pattern Switch and Biomimetic Actuators. Macromol. Rapid Commun. 2020, 41, e2000123. [Google Scholar] [CrossRef]
- Kumar, R. Fabrication and Characterization of Polyvinyl-Alcohol-Based Thin-Film Optical Waveguides. Opt. Eng. 2004, 43, 2134. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.-H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.-J.; Jonas, U.; Wei, T.; Dostalek, J.; Knoll, W. Biosensor Based on Hydrogel Optical Waveguide Spectroscopy. Biosens. Bioelectron. 2010, 25, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Nizamoglu, S.; Gather, M.C.; Humar, M.; Choi, M.; Kim, S.; Kim, K.S.; Hahn, S.K.; Scarcelli, G.; Randolph, M.; Redmond, R.W.; et al. Bioabsorbable Polymer Optical Waveguides for Deep-Tissue Photomedicine. Nat. Commun. 2016, 7, 10374. [Google Scholar] [CrossRef]
- Lakshmipriya, T.; Fujimaki, M.; Gopinath, S.C.B.; Awazu, K.; Horiguchi, Y.; Nagasaki, Y. A High-Performance Waveguide-Mode Biosensor for Detection of Factor IX Using PEG-Based Blocking Agents to Suppress Non-Specific Binding and Improve Sensitivity. Analyst 2013, 138, 2863. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, M.; Yang, C. Fluorescent Hydrogel Waveguide for On-Site Detection of Heavy Metal Ions. Sci. Rep. 2017, 7, e7902. [Google Scholar] [CrossRef]
- Yang, S.; Sarkar, S.; Xie, X.; Li, D.; Chen, J. Application of Optical Hydrogels in Environmental Sensing. Energy Environ. Mater. 2023, 0, e12646. [Google Scholar] [CrossRef]
- Makhsin, S.R.; Goddard, N.J.; Gupta, R.; Gardner, P.; Scully, P.J. Optimization Synthesis and Biosensing Performance of an Acrylate-Based Hydrogel as an Optical Waveguiding Sensing Film. Anal. Chem. 2020, 92, 14907–14914. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable Hydrogel Microbeads for Fluorescence-Based In Vivo Continuous Glucose Monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef]
- Choi, M.; Humar, M.; Kim, S.; Yun, S.-H. Step-Index Optical Fiber Made of Biocompatible Hydrogels. Adv. Mater. 2015, 27, 4081–4086. [Google Scholar] [CrossRef]
- Chen, G.; Hou, K.; Yu, N.; Wei, P.; Chen, T.; Zhang, C.; Wang, S.; Liu, H.; Cao, R.; Zhu, L.; et al. Temperature-Adaptive Hydrogel Optical Waveguide with Soft Tissue-Affinity for Thermal Regulated Interventional Photomedicine. Nat. Commun. 2022, 13, 7789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Layani, M.; Wang, S.; Hu, P.; Ke, Y.; Magdassi, S.; Long, Y. Fully Printed Flexible Smart Hybrid Hydrogels. Adv. Funct. Mater. 2018, 28, 1705365. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Bhusari, S.; Villiou, M.; Pearson, S.; del Campo, A. Printed Degradable Optical Waveguides for Guiding Light into Tissue. Adv. Funct. Mater. 2020, 30, 2004327. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, Q.; Rogin, P.; de Oliveira, P.W.; del Campo, A. Printed Soft Optical Waveguides of PLA Copolymers for Guiding Light into Tissue. ACS Appl. Mater. Interfaces 2020, 12, 20287–20294. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Totaro, M.; Beccai, L. Toward Perceptive Soft Robots: Progress and Challenges. Adv. Sci. 2018, 5, 1800541. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Choi, Y. Soft Optical Waveguide Sensors Tuned by Reflective Pigmentation for Robotic Applications. J. Korea Robot. Soc. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Wallin, T.J.; Pikul, J.; Shepherd, R.F. 3D Printing of Soft Robotic Systems. Nat. Rev. Mater. 2018, 3, 84–100. [Google Scholar] [CrossRef]
- Zhao, H.; O’Brien, K.; Li, S.; Shepherd, R.F. Optoelectronically Innervated Soft Prosthetic Hand via Stretchable Optical Waveguides. Sci. Robot. 2016, 1, eaai7529. [Google Scholar] [CrossRef]
- Williamson, J.G.; Schultz, J. Stretchable Optical Waveguide Sensor Suitability for Wrinkle Degree Detection in Soft Robots. In Proceedings of the 2023 IEEE International Conference on Soft Robotics (RoboSoft), Singapore, 3–7 April 2023; pp. 1–6. [Google Scholar]
- Heiden, A.; Preninger, D.; Lehner, L.; Baumgartner, M.; Drack, M.; Woritzka, E.; Schiller, D.; Gerstmayr, R.; Hartmann, F.; Kaltenbrunner, M. 3D Printing of Resilient Biogels for Omnidirectional and Exteroceptive Soft Actuators. Sci. Robot. 2022, 7, eabk2119. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galindo, J.M.; Tardío, C.; Saikia, B.; Van Cleuvenbergen, S.; Torres-Moya, I. Recent Insights about the Role of Gels in Organic Photonics and Electronics. Gels 2023, 9, 875. https://doi.org/10.3390/gels9110875
Galindo JM, Tardío C, Saikia B, Van Cleuvenbergen S, Torres-Moya I. Recent Insights about the Role of Gels in Organic Photonics and Electronics. Gels. 2023; 9(11):875. https://doi.org/10.3390/gels9110875
Chicago/Turabian StyleGalindo, Josué M., Carlos Tardío, Basanta Saikia, Stijn Van Cleuvenbergen, and Iván Torres-Moya. 2023. "Recent Insights about the Role of Gels in Organic Photonics and Electronics" Gels 9, no. 11: 875. https://doi.org/10.3390/gels9110875
APA StyleGalindo, J. M., Tardío, C., Saikia, B., Van Cleuvenbergen, S., & Torres-Moya, I. (2023). Recent Insights about the Role of Gels in Organic Photonics and Electronics. Gels, 9(11), 875. https://doi.org/10.3390/gels9110875